ΤΗΕ ΑΙ PHARMA MES THAT TRANSFORMS YOUR MANUFACTURING OPERATIONS
Increase process efficiency, product quality, reduce risk and time to market, all in one composable pharma manufacturing execution system.
Trusted by






ΤΗΕ ΑΙ PHARMA MES THAT TRANSFORMS YOUR MANUFACTURING OPERATIONS






COMPLETED PROJECTS
COUNTRIES
CLIENTS
CONNECTED MACHINES
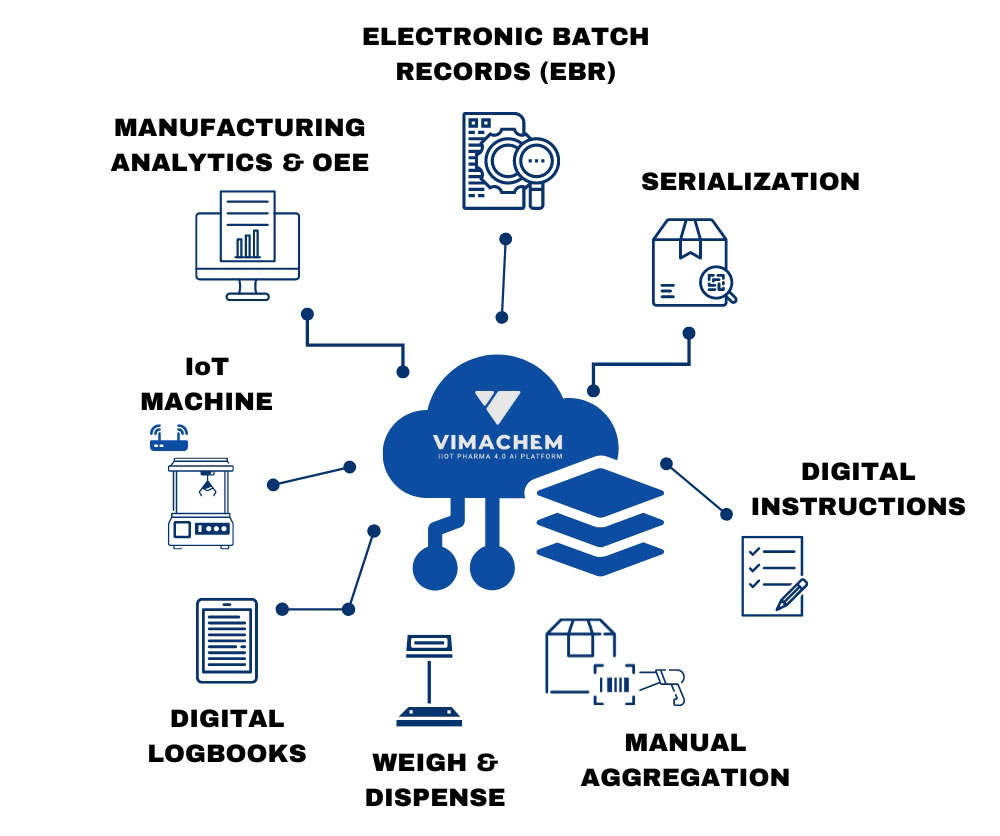
One Modular Platform for Your Pharma/Biopharma Plant


ELECTRONIC BATCH RECORDS (EBR)
A cloud-based digital solution that will help you streamline your production, reduce quality defects and ensure compliance & auditability. Built exclusively for Pharma & Biopharma manufacturers, the software enables faster and more robust implementations.

WEIGH & DISPENSE MODULE (WDM)
A digital solution that enables you to streamline your dispensing activities and ensure compliance & efficiency. Built for Pharma & Biopharma manufacturers, the software is pre-validated & built on modern technology, enabling faster and more robust implementations.

MACHINE CONNECTIVITY
Plug-and-Produce universal machine connectivity automatically collects, transforms, and analyzes data at the source, providing critical real-time visibility to avoid downtime and production losses.

MANUFACTURING ANALYTICS
Multi-tenant cloud platform enables instant manufacturing analytics and insights from anywhere, including OEE machine performance and production monitoring, reporting via APIs, and enterprise tools (ERP, LIMS, APS) integrations.

TRACEABILITY
A cutting-edge traceability software platform, designed in collaboration with Tracelink, allows integration to any Level 2 and corporate Level 4 system, enabling compliance with global traceability standards while optimizing serialization and aggregation operations.

DIGITAL LOGBOOKS
Digitize paper-based logbooks and forms and enable recording and integration of data from equipment, machines, processes, and operators to increase process visibility and comply with cGMP and FDA 21 CFR 211.182.
COMPLETED PROJECTS
COUNTRIES
CLIENTS
CONNECTED MACHINES
TRUSTED BY






Services Dedicated to Pharma Production & Quality
A highly skilled team of consultants, engineers, and computerized system validation (CSV) experts offer specialized services dedicated to the Life Sciences industry.

MES IMPLEMENTATION AND INTEGRATION
We provide high-quality modern software adapted to your needs. Modular build and plenty of add-ons are ensuring fast implementation and immediate return on investment.
LEARN MORE

DIGITAL TRANSFORMATION
We provide a flexible modular Pharma 4.0 MES platform that accelerates your digitization journey to achieve new levels of operational excellence and reduce paperwork.
LEARN MORE

SSM
Vimachem specializes in implementing best-in-class serialization technology to integrate with your existing landscape.
LEARN MORE

COMPUTERIZED SYSTEM VALIDATION AND QUALITY ASSURANCE
We specialize in Computerized System Validation (CSV) and Quality Assurance (QA) support services to our global customers in the life-sciences industry.
LEARN MORE
Dedicated to the Life Sciences industry for 15 years
Our productized services philosophy combines an innovative machine learning driven GxP platform with specialized consulting and computerized system validation (csv) services, that support our Life Science customers in their digital transformation journey and enable them to achieve compliance, improved product quality, production throughout and business excellence.
Read Our Latest News
- 14/11/2025
- 27 secs read
- 16/10/2025
- 21 secs read
- 01/10/2025
- 18 secs read
Services
For Production Optimization & Traceability
Designed specifically for life sciences, Vimachem’s Pharma 4.0 platform brings a unique approach to manufacturing execution systems in the areas of:
- Machine Data Connectivity
- Manufacturing Analytics – OEE
- Serialization Site Manager
- Digital Pharma Logbooks

Ready to Augment your Shop Floor Operations?
Ready to Augment
your Shop Floor
Operations?
Get started with real-time manufacturing analytics today.

















