Τhe ΑΙ Pharma MES That Transforms Your Manufacturing Operations
Vimachem’s all in one composable Pharma Manufacturing Execution System (MES) increases process efficiency and product quality, while reducing risk and time to market.
Trusted by 100+ Pharma & BioPharma Customers










Recognized as the Leading Pharma MES platform!
Trusted for reliability, integration, and customer satisfaction during pharma digital transformation implementations.

4.9
Gartner

4.5
G2
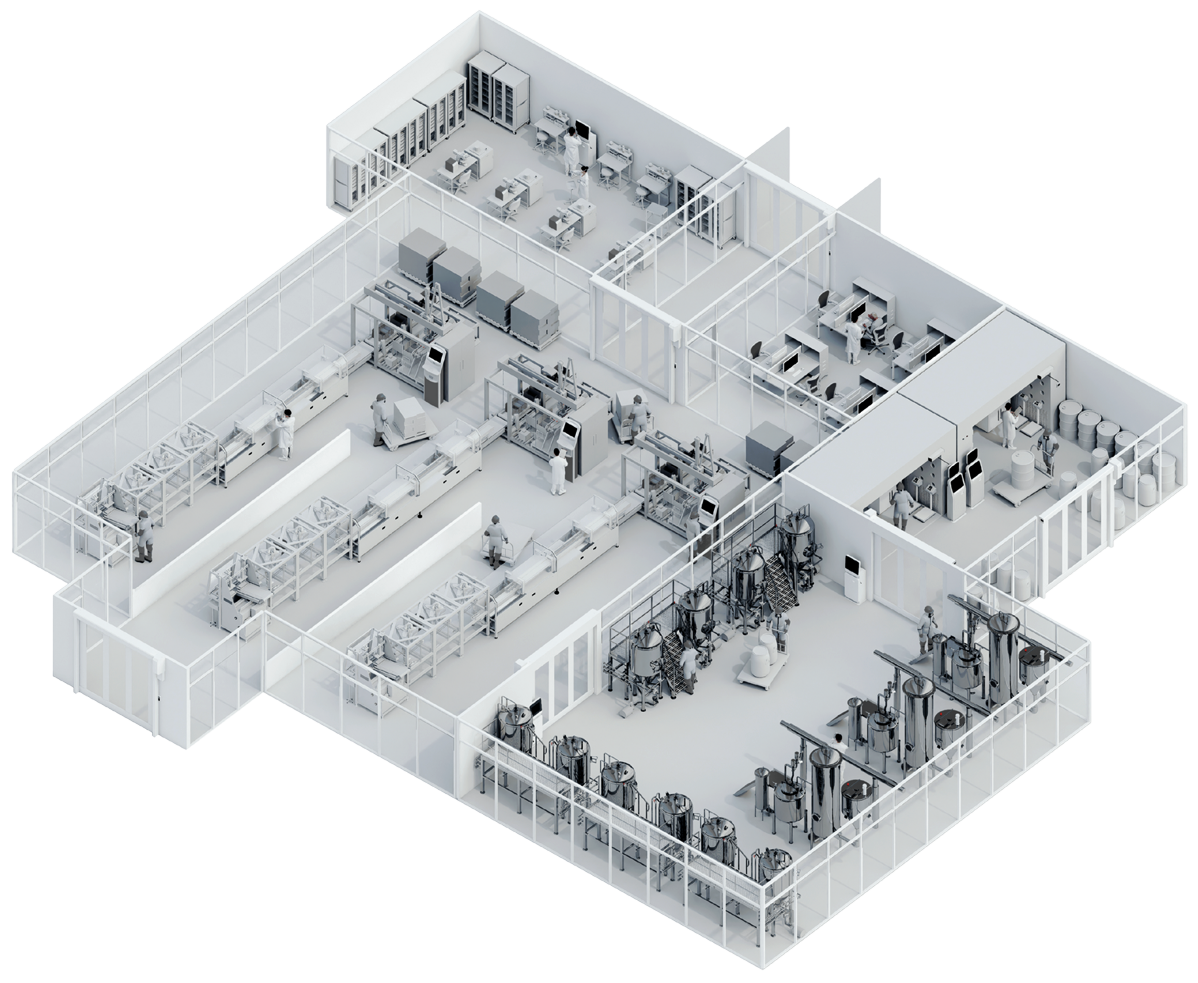
One Modular Platform for Your Pharma/Biopharma Plant
Visibility | Quality | Efficiency
- Gain a single, real-time view of batches, equipment utilization, and operator activities across the shop floor.
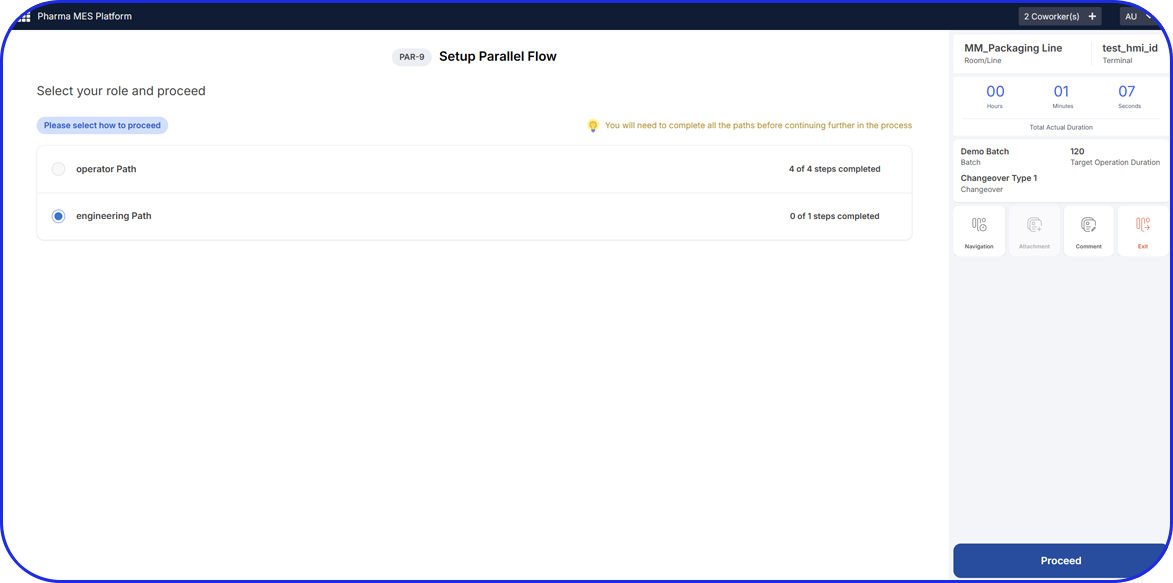
- Guide operators with validated, GxP-compliant workflows that enforce right-first-time execution and electronic sign-offs.
- Automate data capture from equipment, sensors, and integrated systems to ensure complete traceability and data integrity.
- Centralize quality and performance insights to eliminate manual delays, deviations, and inefficiencies.
Services Dedicated to Pharma Production & Quality
A highly skilled team of consultants, engineers, and computerized system validation (CSV) experts offer specialized services dedicated to the Life Sciences industry. Drawing on deep expertise in Manufacturing Execution Systems (MES), our team supports digital transformation initiatives that drive operational excellence and compliance.

MES Implementation and Integration
Vimachem delivers high-quality, modern software tailored to your needs. Our modular design and extensive range of add-ons enable rapid implementation and drive immediate ROI.

Digital Transformation
Vimachem offers a flexible, modular Pharma 4.0 MES platform that accelerates digital transformation, reduces paperwork, and drives operational excellence.

SSM
Vimachem specializes in implementing best-in-class serialization technology to seamlessly integrate with your existing landscape and workflows.

Computerized System Validation and Quality Assurance
Vimachem specializes in Computerized System Validation (CSV) and Quality Assurance (QA) support services for our global customers in the life-sciences industry.
Dedicated to the Life Sciences industry for 15 years
Vimachem’s productized services philosophy combines an innovative, machine learning–driven GxP platform with specialized consulting and Computerized System Validation (CSV) services—supporting life sciences customers on their digital transformation journey while enabling compliance, improved product quality, increased production throughput, and overall business excellence.
Read Our Latest News
- 23/12/2025
- 12 secs read
- 14/11/2025
- 27 secs read
- 16/10/2025
- 21 secs read
Ready to Augment your Shop Floor Operations?
Ready to Augment
your Shop Floor
Operations?
Get started with real-time manufacturing analytics today.