OEE Fundamentals
OEE Fundamentals

Olga-Maria Plessa
Product Owner / Lead Consultant
What is OEE and why is it important in pharmaceutical manufacturing?

Fundamentals of OEE= availability, performance and quality
Overall Equipment Effectiveness (OEE) Overview
In an ideal pharmaceutical or biotech factory, equipment would operate 100% of the available time, at 100% capacity based on machines’ validated performance, and with an output of 100% quality products.
In reality however, there are losses and stops during production that can be caused by several parameters such as machine failures or underperformance or quality issues, and those parameters can be linked to financial KPIs directly impacting profitability.
What is OEE For?
With the Overall equipment effectiveness (OEE) KPI, pharmaceutical manufacturers can evaluate how effectively manufacturing operations are executed.
Through OEE tool, root causes can be detected and action can be decided to analyze and improve processes.
By using machine sensors, OEE data collection can be done in real-time by providing an efficient way to monitor process-related downtimes and enable immediate improvement in efficiency and machine performance.
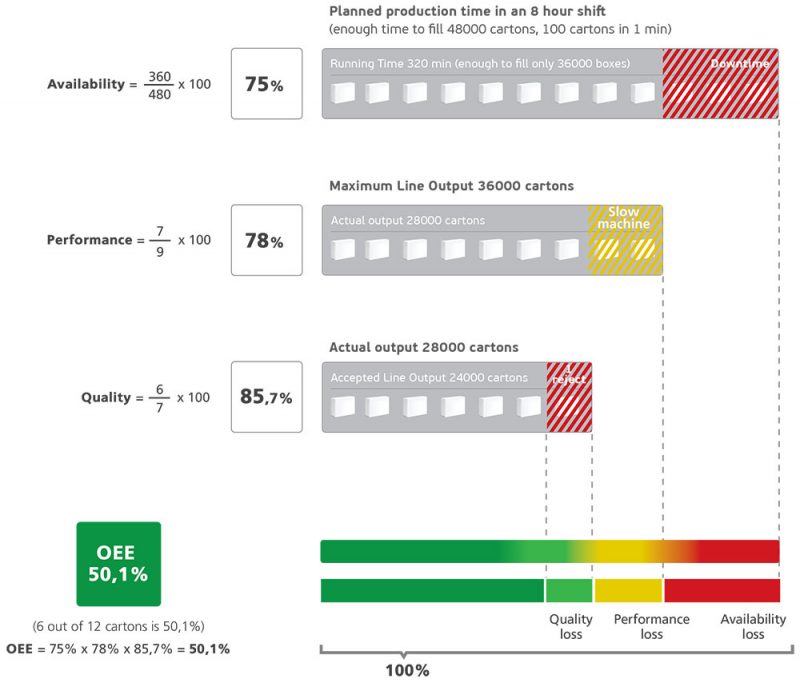
OEE Calculation
OEE or Overall Equipment Effectiveness is a ratio of how much a pharmaceutical manufacturing facility is utilized for, compared to the full output it was designed for.
In other words, it is a measurement of productivity that calculates the efficiency at which equipment can produce finished goods, by considering the three fundamentals of OEE:
OEE Fundamentals

Availability, Performance and Quality
Availability of OEE
Availability is the uptime, meaning the total time the pharmaceutical manufacturing facility is available for, minus maintenance and unscheduled downtimes.
Availability is the percentage of planned production time that can be allocated for manufacturing or in other words is the percentage of scheduled time that the operation is available to operate. The losses in this category are unplanned and planned stops.
Unplanned stops consist of the time where equipment is scheduled to produce but is not running due to unplanned occurrences such as equipment breakdowns, unplanned maintenance or materials shortage.
On the other hand, planned stops take into account the time where equipment is scheduled to produce but is not running due to planned occurrences such as changeover, tooling adjustment, cleaning, planned maintenance and quality inspections.
Performance of OEE
Performance is the actual throughput of the plant during the time it runs, compared to the maximum throughput that it could have achieved running at the validated speed. We categorize losses as micro stops and slow cycles.
Micro stops are the time when the equipment stops for a very short period (typically 1 – 2 minutes or less) with the operator typically resolving the issue. Some examples of micro stops are material jams, incorrect settings on the machine, misaligned or blocked sensors, equipment design issues, and periodic quick cleaning due to misfeeds.
Slow cycles are the time when equipment runs slower than the validated speed. This might be a consequence of not properly cleaned equipment, poor machine lubrication, substandard materials, poor environmental conditions, wrong settings/adjustment in PLC or other human factors such as training, experience and internal processes.
Quality of OEE
Quality is the number of actual production output, which meets customer specifications and has no defect (good units) on its first pass through a line (first pass yield) compared to total products that are being produced.
Quality can be lost in two ways – by producing rejects, or start-up rejects that exist at the beginning of any given process.
Production rejects are defects that we have produced during stable production – including those that we can rework as OEE measures quality based on Right First Time”. Examples include under or overweight bags, label problems, chemical or physical conformity issues, broken packaging and more.
Start-up rejects are defects that we have produced from start-up until stable production. They can occur after any equipment start-up. However, most of the times shop floor teams tend to track them after changeovers.
Examples include suboptimal changeovers, equipment that needs “warm-up” cycles, or equipment that inherently creates waste after start-up.
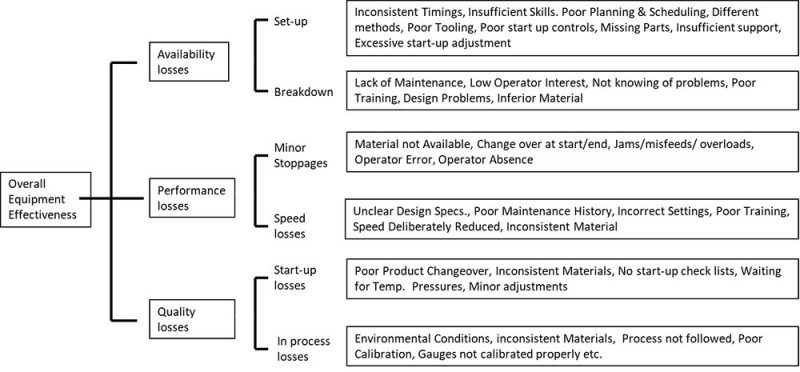
Each of the OEE parameters is furtherly broken into subcategories to identify the root-cause of downtimes and plan on how to improve or eliminate them, if possible.
The Value of Overall Equipment Effectiveness (OEE)
Calculating OEE in Pharmaceutical Manufacturing is critical to profitability!
Overall equipment effectiveness (OEE) monitoring and optimization is critical as the market leading pharmaceutical manufacturers are 53% faster in getting product to the market.
OEE data helps in data-driven decisions for:
- Investments in operators training.
- Investments in manufacturing infrastructure.
- Enabling Continuous Improvement and Lean Manufacturing value.
- Calculating ROI and profit gain from process and infrastructure improvement.
- Comparing Investments through accurate ROI data.
- Minimizing efforts to follow up production performance.
- Digitizing plant and getting quick and reliable data.
- Increasing and securing manufacturing output.
- Getting full control over operations and machine performance.
Ready to Augment your Shop Floor Operations?
Ready to Augment your Shop Floor Operations?
Vimachem Manufacturing Analytics - OEE
The Manufacturing Analytics – OEE module is an intelligent Pharma OEE Cloud solution that allows you to collect, store and visualize data across your site / enterprise and apply AI algorithms to optimize production efficiency and product quality.










