From Paper-Based to Scalable Serialization! How Fulton CDMO Standardized on Vimachem Level 3 SSM & Manual Aggregation Module to Enable Compliance and Growth From Paper-Based to Scalable Serialization! How Fulton CDMO Standardized on Vimachem Level 3 SSM & Manual Aggregation Module to Enable Compliance and Growth Trusted by 100+ Pharma & BioPharma Customers Company Profile […]
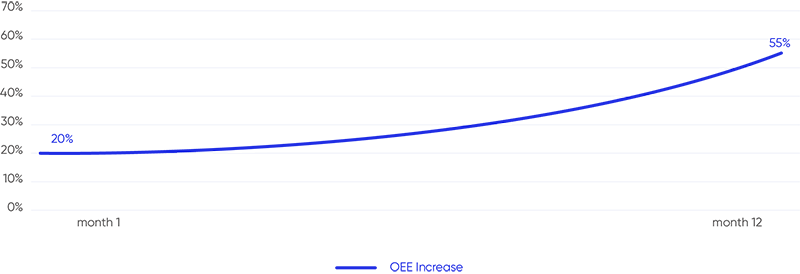
How ELPEN Increased OEE by 90% and achieved 299% ROI with Vimachem’s Manufacturing Analytics & OEE solution How ELPEN Increased OEE by 90% and achieved 299% ROI with Vimachem’s Manufacturing Analytics & OEE solution Labatec Achieves a 35% OEE Increase and achieved 324% ROI with Vimachem’s Manufacturing Analytics and OEE Solution Labatec Achieves a 35% […]
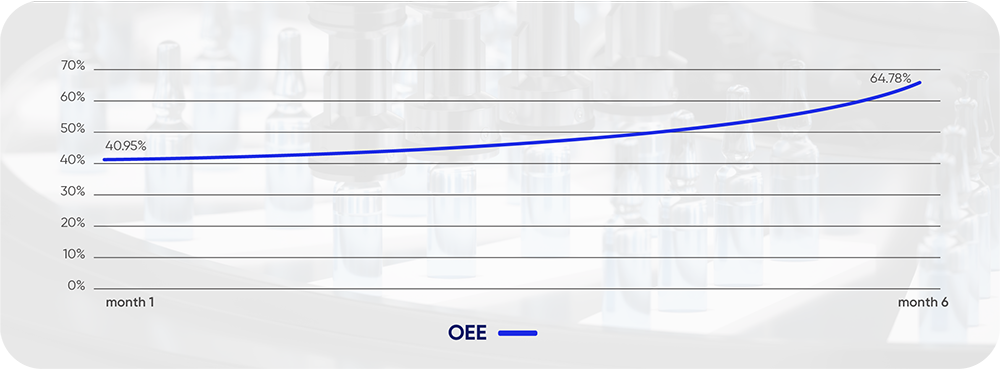
From 41% to 65% OEE! A 59% Increase – Inside Astrea Pharma’s OEE Breakthrough on their First Production Line with Vimachem’s MA & OEE. From 41% to 65% OEE! A 59% Increase – Inside Astrea Pharma’s OEE Breakthrough on Their First Production Line and How They Subsequently Scaled Across All Lines with Vimachem’s MA&OEE Company […]
How ELPEN Increased OEE by 90% and achieved 299% ROI with Vimachem’s Manufacturing Analytics & OEE solution How ELPEN Increased OEE by 90% and achieved 299% ROI with Vimachem’s Manufacturing Analytics & OEE solution Company Profile Organization: ELPEN • Company Size: 1450+ employees • Location: Greece, operating in 90+ countries • Industry: Pharmaceutical manufacturing www.elpen.gr […]
// Webinar Ensure On-Time Drug Shipments: How to Quickly Replace Your Serialization System with the Leading Track-and-Trace Platform In 2019, Help Pharmaceuticals implemented a new serialization solution from another provider to comply with the EU Falsified Medicines Directive. However, the solution’s closed architecture required complex and difficult-to-manage interfaces which complicated the process of onboarding new […]
// Webinar Serialization Compliance in Russia with Medice and Wipotec The specifics of the Russian system and how to meet the requirements In cooperation with our customer Medice and our L1-2 partner Wipotec, our experts explain the special challenges of serialization in the Russian system. Producers from all industries must meet strict requirements when using […]
// FRESENIUS KABI Case Study Serialization Driving Innovation & Change With RFID Fresenius Kabi to optimally support the US healthcare system, decided to implement RFID (Radio Frequency Identification) inventory tracking systems for pharmaceutical products sold in US hospitals. Without RFID, the process of identifying what products are in the hospital and how many, is very […]
// ASPEN Case Study Serialization For Global Medicines Traceability Aspen Australia, one of the largest pharmaceutical companies in Australia, needed to face the ever-changing regulatory requirements that European Commission (EU) created by developing the Falsified Medicines Directive (FMD) which requires serial numbers to be printed onto pharmaceutical products and for product serialization data to be […]
// IBERFAR Case Study Pharma Trackts 2021 | Serialization Driving Change And Innovation Iberfar a global pharma contract manufacturer (CMO) needed an open and flexible serialization software solution to be able to support global customer serialization requirements including installation of aggregation equipment and exports to the Russian market. As a result, the Vimachem Level 3 […]