Serialization For Global Medicines Traceability
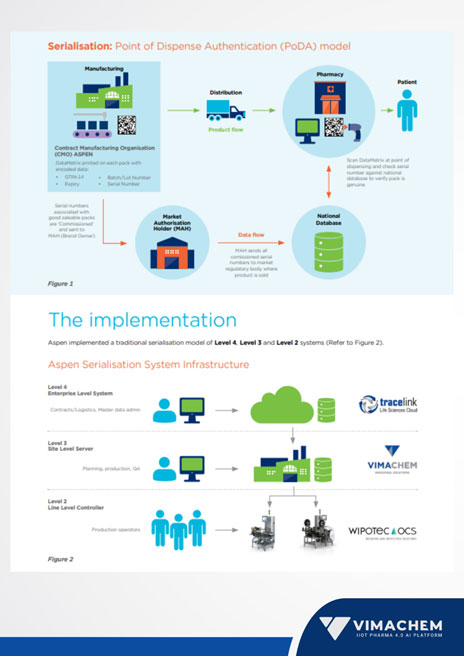
Aspen Australia, one of the largest pharmaceutical companies in Australia, needed to face the ever-changing regulatory requirements that European Commission (EU) created by developing the Falsified Medicines Directive (FMD) which requires serial numbers to be printed onto pharmaceutical products and for product serialization data to be transferred from the manufacturer to the regulator. To meet the anti-counterfeiting requirements of their target markets, in late 2018 Aspen embarked on the journey toward implementing a traditional serialization model of Level 4, Level 3, and Level 2 systems.
Vimachem SSM (Level 3) was best aligned with Aspen’s selection criteria, as it seamlessly integrates with Tracelink (Level 4) while having a universal (non-proprietary) interface with the Wipotec-OCS (Level 2) serialization and aggregation equipment. The successful implementation was led by our SSM project team who remotely managed the complete implementation, provided all qualification documentation, and executed in collaboration with the Aspen team, the validation of both Test and Production environments.
The serialization system’s commercial introduction took place in July 2020. Using GS1 DataMatrix and EPCIS standards, the implementation has effectively allowed Aspen to supply serialized products into export markets that are entirely compliant with their regulatory requirements. The challenge of counterfeit medications on international markets is addressed by using worldwide standards for serialization, which also facilitates tracking.
The benefits of this implementation were:
- One best-of-breed (Wipotec-Vimachem- Tracelink) solution for ten different export markets
- Fully compliant with FDA CFR 21 part 11
- Implementing global standards enables traceability
- Combats counterfeit products