From 41% to 65% OEE! A 59% Increase - Inside Astrea Pharma's OEE Breakthrough on their First Production Line with Vimachem's MA & OEE.
From 41% to 65% OEE! A 59% Increase – Inside Astrea Pharma’s OEE Breakthrough on Their First Production Line and How They Subsequently Scaled Across All Lines with Vimachem’s MA&OEE
Company Profile
Organization: Astrea Pharma • Company Size: 230 employees • Location: Fontaine-lès-Dijon and Monts, France • Industry: Pharmaceutical manufacturing
Astrea Pharma is a contract development and manufacturing organization (CDMO) operating a flagship facility in Fontaine-lès-Dijon, France. The site produces oral solid dosage forms (tablets, capsules, powders) and offers both aseptic manufacturing and clinical supply support. Astrea recently expanded into Monts in France, where its injectable forms are manufactured, to serve growing biopharma needs, reinforcing its commitment to operational excellence and data-driven production.

Production Overview
Astrea’s Fontaine-lès-Dijon facility includes six packaging lines: five for blister packaging and one bottling line. The workshop runs across three shifts and produced 58 million boxes in 2024. Prior to implementing Vimachem, data collection was manual, with operators filling in paper sheets and Excel files. This led to reliability issues, especially during night shifts, and created obstacles for performance tracking, loss analysis, and continuous improvement.
The Challenge
Astrea faced several key operational challenges:
- Lack of real-time production visibility for supervisors and management.
- Manual data collection prone to inconsistency and human error.
- Missing or incomplete downtime data, limiting root cause analysis.
- Difficulties quantifying and prioritizing loss areas.
- Fatigue and low engagement among operators due to manual reporting.
- Delayed or missed weekly performance reviews.
- No clear structure to identify or measure progress toward performance improvement.
Astrea needed an intuitive, scalable solution that could capture real-time production data without interrupting operations or requiring major infrastructure changes.
In January 2024, Astrea implemented Vimachem’s Manufacturing Analytics and OEE solution, beginning with a light version and progressively rolling out the full operator interface line-by-line. Integration with existing serialization systems enabled real-time data capture with no disruption to packaging lines.
Key Features Used:
- Real-Time OEE Monitoring: Tracking availability, performance, quality, and stoppages.
- Paperless Data Capture: Eliminated operator fatigue from manual logs.
- ERP Integration: Automated upload of process orders to streamline planning.
- Line-Level Dashboards: Provided line managers with visibility into weekly trends.
- Progressive Rollout: Deployed on six packaging lines from September 2024 through early 2025.
The Solution
Implementation Process
Astrea deployed Vimachem's OEE solution gradually, beginning in January 2024, with remote support from Vimachem:
Phase 1: Light Solution
- Basic output data collected from existing serialization systems.
- No automation engineer or hardware changes required.
- Enabled immediate productivity visibility without disrupting operations.
Phase 2: Full Interface Rollout
- From September 2024, one packaging line was upgraded per month.
- All six lines - five blistering, one bottling - now use the full operator interface.
- ERP integration enabled automatic process order uploads, significantly reducing planning time.
- Bi-weekly Analysis: Astrea and Vimachem met regularly to analyze performance and guide improvements.

The Results
Astrea has seen continuous and measurable improvements since implementing Vimachem:
• Partena 4 Line:
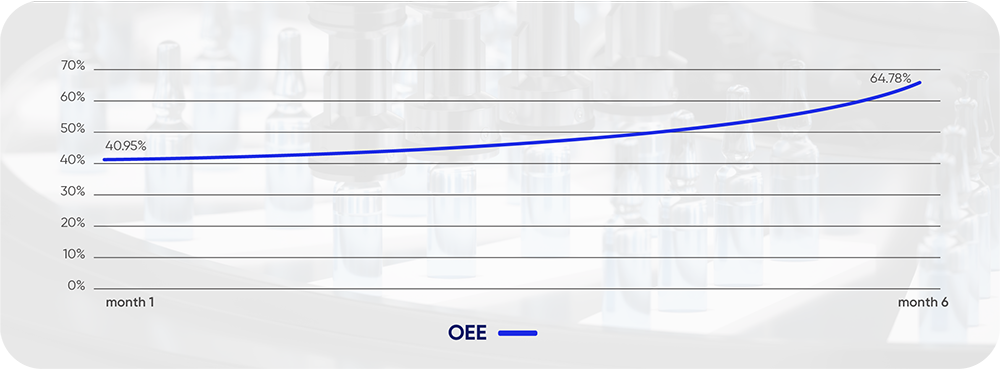
- September 2024 OEE: 41%.
- Consistent Performance: Sustained 65% OEE for three consecutive months (March 2025 output: - 69,000 boxes/day over 3 shifts), demonstrating stable, repeatable results.
- 59% increase.
- Enabled immediate productivity visibility without disrupting operations.
• Partena 1 Line:
- Achieved similar steady gains after its deployment in November 2024.
• Weekly Action Planning:
- Based on reliable, complete operator and system data.
• Executive Visibility:
- Dashboards allow top management to monitor performance remotely, in real time.
OEE
Operational Gains
- Real-Time Dashboards: Provided clarity and accountability across shifts.
- Reduction in Planning Effort: ERP integration eliminated scheduling delays.
- Changeover Optimization: More accurate tracking led to improved processes.
- Operator Mindset Shift: From seeing the system as oversight to seeing it as a tool.
- Performance-Driven Culture: Weekly reviews and trend data now drive continuous improvement.
OEE Growth
Key Performance Indicators (KPIs)
- Downtime Insight: Shift-by-shift tracking enabled root cause analysis.
- OEE Increase (Partena 4): From 41% to 65% (+59% relative increase).
- Monthly OEE Growth: average of 8% per month.
- Packaging Output: 58 million units produced in 2024.
- Planning Time Reduction: Significant time savings due to ERP integration.
- Operator Engagement: High adoption rate and increased enthusiasm across all six lines.

Key Benefits of Vimachem's Manufacturing Analytics & OEE Module
- Remote & Rapid Deployment: Zero disruption to production.
- No Line Changes Required: Seamless integration with existing systems.
- Intuitive Interface: High adoption among operators.
- Configurable Dashboards: Customized for roles and responsibilities.
- Continuous Support: Bi-weekly sessions with Vimachem to track progress.
Conclusion
Astrea Pharma’s adoption of Vimachem’s solution has delivered significant and sustained operational improvements. With a modernized, data-driven packaging workshop, they have increased efficiency, empowered operators, and enabled real-time decision-making across the business.
Ready to Augment your Shop Floor Operations?
Ready to Augment your Shop Floor Operations?
Get started with real-time manufacturing analytics today.










