From Paper-Based to Scalable Serialization! How Fulton CDMO Standardized on Vimachem Level 3 SSM & Manual Aggregation Module to Enable Compliance and Growth
From Paper-Based to Scalable Serialization! How Fulton CDMO Standardized on Vimachem Level 3 SSM & Manual Aggregation Module to Enable Compliance and Growth
Trusted by 100+ Pharma & BioPharma Customers










Company Profile

Organization: Fulton Medicinali (CDMO) • Company Size: 50-200 employees • Location: Arese (Milan area), Italy • Industry: Pharmaceutical Manufacturing (CDMO)
"Serialization can be complicated. We needed components that work together easily and a vendor we could rely on."
Luca Giossi, QP
Fulton Medicinali is a contract development and manufacturing organization (CDMO) specializing in suppositories and ovules, with additional capabilities in solid dose forms such as tablets and capsules. Operating from a single site near Milan, Fulton serves approximately 50 active customers worldwide and maintains a small branded portfolio for the Italian market. With a lean organizational structure, the company’s Qualified Person (QP) directly oversees serialization and digitalization initiatives.
Production Overview
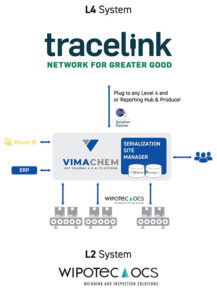
Fulton operates two packaging lines, with a third Wipotec line currently being installed to support growing volumes. Serialization and aggregation are managed through Vimachem’s SSM (Level 3) platform, supported by the MAM (Manual Aggregation Module). The site uses Wipotec equipment for serialization and TraceLink at Level 4 for regulatory messaging. Given the current shipment frequency, Level 4 messaging is still handled manually, with automation planned as demand scales.
The Challenge
Managing CDMO complexity during Italy’s serialization transition
- Multi-client environment: Fulton needed to manage multiple customers with different serialization requirements, frequent changeovers, and strict compliance demands.
- Regulatory burden: Italy’s coexistence of the legacy Bollino label and the EU FMD safety features increased operational complexity and costs.
- Limited IT resources: With no internal IT department, the company required a solution that was intuitive, low-maintenance, and manageable by the QP and operators.
- Growth readiness: The upcoming installation of a third packaging line required a system that could scale quickly and integrate seamlessly with new equipment.
Vimachem SSM (Site Manager) and MAM (Manual Aggregation Module)
- Ease of use: Operators described the system as straightforward and “very smooth” in daily operations.
- Fast implementation: Production-ready go-live completed in just 12 weeks, with a smooth qualification process and no disruption to production.
- Low maintenance: The QP manages the system directly, with minimal support needed from external resources.
- Integration-ready: Proven compatibility with TraceLink (L4) for regulatory reporting and Wipotec line equipment for expansion.
- Flexible approach: MAM supports compliant manual aggregation where automation is not yet required, with a clear path to automated messaging when volumes increase.
Implementation Process
The full production-ready system was deployed in just 12 weeks, aligning with Fulton’s scale-up timeline and ensuring uninterrupted packaging operations.
1. Setup & Design:
SSM configured to meet Fulton’s CDMO workflows; MAM enabled for manual aggregation.
2. Operator Training:
Fast onboarding; operators quickly adapted to the system.
3. Go-Live & Qualification:
Smooth process, with no major issues and expectations consistently met.
4. Expansion Readiness:
Proactive work completed ahead of Wipotec machine delivery to accelerate the third line’s startup.
"I had used Vimachem before and trusted the solution. I was given many other options to pursue but I wanted to remain with a vendor I had tried and trusted."
Luca Giossi, QP
The Results
Operational benefits achieved to date:
- Stable, compliant serialization supporting Fulton’s largest global customer.
- Strong operator adoption with minimal training required.
- Confidence to expand serialization seamlessly to a third packaging line.
- Clear roadmap to transition from manual to automated L4 messaging as shipping volumes grow.
- Reliable vendor support and a partnership-oriented commercial approach.
Performance Highlights:
- Faster line changeovers for serialized SKUs, improving flexibility across customer projects.
- Time saved in audit preparation for QA/QP through simplified documentation and traceability.
- Fewer L4 message exceptions and reduced manual handling following automation, increasing data accuracy and batch release efficiency.
- Exceptionally low support load: Only 7 support tickets raised over two years, demonstrating high system stability and ease of maintenance.
Operational Gains:
- Operator efficiency: User-friendly interface requiring minimal training.
- Qualification simplicity: Described as easy, with no significant issues.
- Agility: Faster onboarding of new SKUs and customers across multiple markets.
- Scalability: Prepared for seamless expansion to the third line and beyond.
- Compliance confidence: Reliable system performance through Italy’s regulatory transition.
Key Benefits of Vimachem SSM & MAM
- Rapid deployment without disrupting production.
- Minimal IT requirements, enabling direct management by QA/QP.
- Line-agnostic architecture, integrating with both existing and new equipment.
- Flexible workflows suited to CDMO operations and audit needs.
- Future-proof design, enabling a smooth path from manual to automated data flows.
Conclusion
Fulton Medicinali’s adoption of Vimachem’s SSM & MAM has enabled the company to manage serialization with ease despite regulatory complexity and limited IT resources. By combining operator-friendly design, reliable implementation, and scalability, Fulton is well-positioned to expand serialization to new lines and customers while maintaining compliance and operational efficiency.
Ready to Augment your Shop Floor Operations?
Ready to Augment your Shop Floor Operations?
Get started with real-time manufacturing analytics today.