Should you integrate Electronic Logbooks and OEE?
Should you integrate Electronic Logbooks and OEE?

Łukasz Iskra
Business Development Director
As someone who regularly supports pharma and biopharma companies – especially CDMOs, CPOs – on their digital transformation journey, I often get the same question:
“Should we integrate Electronic Logbooks with OEE data?”
My answer is: absolutely yes – and here’s why.
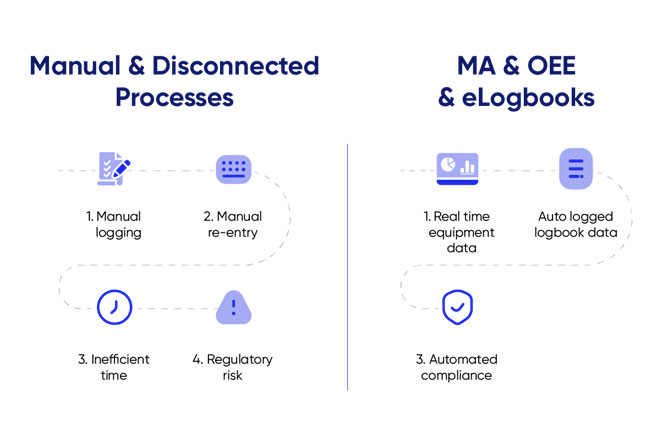
The reality today
Many pharma companies still rely on paper-based or disconnected systems for OEE monitoring and logbook documentation.
This means:
- Operators are manually writing entries.
- QA teams spend hours manually verifying data integrity.
- Operations waste time duplicating data between disconnected systems.
- Root cause analysis during audits causes unnecessary delays.
Integration Improves Quality and Reduces Risks
Integrating OEE and Electronic Logbooks within a modular Pharma MES empowers operators to focus on high-quality production, and QA teams can prioritize oversight of high-risk operations:
1. Automatic logbook entries are triggered based on real-time OEE events.
A downtime of over 15 minutes automatically creates a logbook entry for the equipment, with the operator ID, product code, start date and time, and line ID prefilled.
No duplication – the OEE system already captures:
- Product Code
- Product Name
- Operator ID
- Line Status
- Start and Stop Times
- Stop Reason
So why require the operator to re-enter information that the system already captures automatically?
2. Equipment State Awareness in both Logbooks entries and OEE Monitoring
The Logbook Designer pulls contextual data, such as equipment and cleaning statuses, reducing reliance on manual inputs. This enables operators to avoid unnecessary cleaning steps and reduces the risk of human error, helping to minimize process deviations and ensure compliance. Equipment statuses are automatically updated following maintenance activities or batch execution, fostering real-time coordination and alignment between QA, Maintenance, and Production teams.
3. Both modules as part of the Pharma specific MES platform
Each action is fully audit-trailed, timestamped, and linked to user credentials and production batches, ensuring data integrity and traceability at every step. The seamless integration between modules streamlines batch execution and logbook completion, significantly reducing compliance risk. Once data is captured, logbook entries can follow a configurable multi-level approval workflow with secure e-signatures.
Unlike generic platforms, Vimachem delivers:
- Pre-built integration logic between MA&OEE and Digital Logbooks.
- No-code logbook design using validation-ready templates.
- GAMP 5 and CSA-aligned validation toolkit with automated evidence capture.
- ALCOA+ and Annex 11 compliance out of the box.
This reduces validation workload and eliminates deployment delays, enabling most customers to go live in weeks, not months.
What about Validation?
Validation is essential in GMP-compliant manufacturing. Because the OEE system is already validated and communicates validated data to the electronic logbook through a validated interface, it significantly reduces the validation burden for the logbook module.
Vimachem provides a pre-validated platform, complete with a GAMP-compliant validation package, ready for risk assessment and execution – streamlining implementation while ensuring compliance.
This ensures:
- Traceability for every field entry.
- GMP-compliant automation.
- Full alignment with 21 CFR Part 11 and EU GMP Annex 11.
- Reduced risk of human error.
- Less time wasted in documentation reviews and audits.
Use Case: Packaging Line Efficiency Boost
In a recent Vimachem deployment:
- A CDMO integrated OEE with logbooks on its blister packaging line.
- Equipment downtime, cleaning logs, and shift events were auto-captured.
- Paper logbooks were eliminated entirely within 6 weeks.
- OEE improved by 9% in 3 months – with zero deviation due to missing documentation.
Why it matters
Integrating OEE and eLogbooks helps Pharma / Biopharma manufacturers:
- Secure compliance and audit readiness.
- Gain efficiency by eliminating paper or double work.
- Secure data integrity by relying on machine-logged, timestamped information.
- Drive continuous improvement with better visibility into operations.
- Empower QA, MES, and Operational Excellence teams with real-time, reliable data.
Vimachem helps pharma and biopharma manufacturers connect machines and fully digitize their OEE + Logbook workflows.
Book a demo to see how it works in one integrated modular MES Platform.
Take the first step towards eliminating paperwork, reducing deviation risks, and gaining full control of your production floor.










