ERP Can't Weigh Your Batches: The Case for MES-Native Weigh & Dispense
ERP Can't Weigh Your Batches: The Case for MES-Native Weigh & Dispense

Emma Hanley
Senior Product Marketing Manager
Introduction
In pharmaceutical manufacturing, precision is everything. Every gram matter, every action must be traceable, and every record must withstand regulatory scrutiny. Yet many companies still assume their enterprise resource planning (ERP) system can cover all areas of their operations – including critical GMP processes like weighing and dispensing (W&D).
This assumption is risky. ERP systems excel at planning and financial oversight, but they are not designed for shop-floor execution in highly regulated environments. When W&D is forced into ERP, manufacturers often face compliance gaps, inefficiencies, and costly workarounds.
It’s time to ask: should your W&D really live inside your ERP?
ERP's Strengths - and Its Limits
ERP is indispensable in pharma for:
- Managing enterprise-level resources and planning.
- Overseeing procurement, finance, and supply chain.
- High-level visibility of materials and operations.
But ERP was never built to handle tasks like:
- Real-time integration with industrial scales, barcode scanners , or dispensing equipment.
- Recipe enforcement and weighing tolerances at the operator level.
- Seamless integration with Electronic Batch Records and Electronic Logbooks.
These gaps are critical when it comes to W&D, where compliance and accuracy are non-negotiable.
Why ERP Struggles with Weighing & Dispensing
- Designed for Office Use not for Pharmaceutical Shopfloors
ERPs are designed for planners, not shop-floor operators. Forcing operators to use ERP for weighing tasks increases training time, errors, and frustration. - Heavy Customization = Validation Complexity
Customizing ERP to handle W&D introduces validation burdens. Every system update requires re-validation, adding cost and complexity while creating risk during audits. - Inadequate Traceability and Documentation:
The pharmaceutical industry requires meticulous, tamper-proof documentation for every single gram of material. A Manufacturing Execution System (MES) automatically generates a detailed electronic batch record (eBR), capturing every action, from the exact dispense time to the operator involved and the equipment used. This ensures end-to-end traceability, ensuring full compliance during regulatory audits. By contrast, an ERP’s primary function is not to create this level of detailed, real-time documentation.
The Risks of Keeping W&D in ERP
When pharma manufacturers force ERP to cover W&D, they risk:
- Regulatory non compliance: incomplete audit trails and gaps in data integrity undermine credibility, and can lead to findings during inspections, warning letters, or even stop production.
- Operational inefficiencies: without direct equipment connectivity, manual handoffs and data entry increase the risk of costly errors and batch failures.
- Hidden costs: extensive ERP customizations increase long-term support, maintenance, and re-validation expenses.
- Constrained innovation: extensive custom coding increases the complexity and risk of system upgrades, often resulting in disruption to operations and delayed improvements.
- Lack of Real-Time Control: Weigh & Dispense requires immediate communication with scales and barcode scanners, streaming data as it happens. ERP, as a transactional system, only logs data after the fact and cannot actively control the weighing process.
- Slower operations: ERP transactions are heavier and less responsive, creating bottlenecks for operators during weighing.
- Higher error rate: ERP screens are not designed for guided execution. Without step-by-step instructions, operators risk weighing the wrong material or incorrect quantity.
The net effect: higher risk, higher cost, and lower agility.
Why MES W&D purpose-built for Pharma Is a Better Solution
A Pharma Manufacturing Execution System (MES) with a dedicated W&D module is built for pharmaceutical weighing operations as it offers:
- Real-time equipment integration: balances, scanners, and dispensing devices connect seamlessly.
- Operator-friendly workflows: guided steps reduce errors and training needs.
- Precision and control: recipe management, material tracking, and tolerance checks.
- Simplified validation: MES updates are scoped and purpose-built for regulated use.
- Seamless execution: W&D is the first step in batch execution, fully embedded within the MES and interoperable with the Electronic Batch Record (EBR) and electronic logbooks. This ensures complete traceability across materials, equipment, and operators.
Rather than overburdening ERP, MES complements it – ensuring W&D is accurate, compliant, and efficient.
The Best of Breed Model: ERP + MES
The future-ready pharma architecture isn’t ERP-only. It’s ERP + MES, each system doing what it does best:
- ERP manages enterprise planning and resources.
- MES-native Weigh & Dispense ensures compliance and execution on the shop floor.
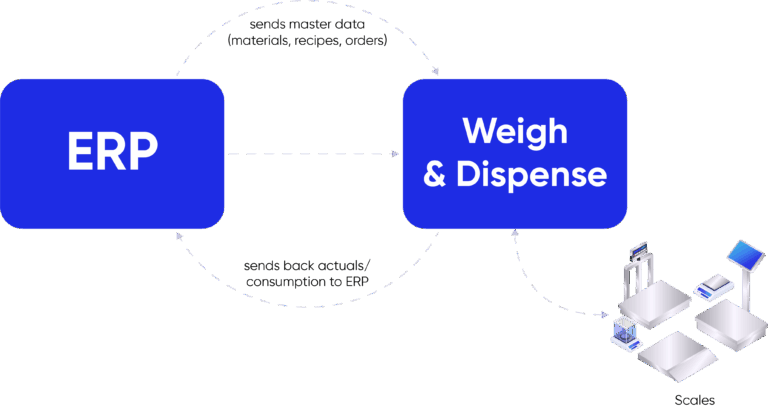
This best-of-breed approach protects compliance, reduces cost, and drives digital maturity. Its real strength is the seamless integration of ERP and MES, which aligns planning and execution into one continuous process. ERP provides production orders, BOM references, material data, and target quantities that MES uses to initiate weighing and dispensing. MES then manages execution in real time – guiding operators, interfacing with balances and scanners, enforcing tolerances, and capturing every action in the eBR and logbooks. Once weighing is complete, MES returns actual consumption, yields, variances, and status updates back to ERP, keeping inventory and planning fully aligned.
The result is a closed-loop model that unites enterprise planning with compliant shop-floor execution – eliminating manual handoffs, reducing errors, and ensuring both operational efficiency and regulatory confidence.
Conclusion
ERP is essential – but it was never meant to manage critical GMP shop-floor processes. For weighing and dispensing, the stakes are too high: compliance, accuracy, and efficiency cannot be left to chance.
By moving W&D into MES, pharma manufacturers safeguard compliance, empower operators, and avoid the cost traps of ERP customization.










