MES-EBR Integration: What Pharma Manufacturers Really Mean - and How You Should Actually Implement It
MES-EBR Integration: What Pharma Manufacturers Really Mean - and How You Should Actually Implement It
Implementing an Electronic Batch Record (EBR) within your Manufacturing Execution System (MES) revolutionizes pharmaceutical manufacturing.
This leading-edge approach creates a centralized, real-time data hub, offering pharma manufacturing considerable advantages.
But, many pharma and life sciences manufacturers researching digital transformation start by looking for an MES-EBR integration. And while this search term is common, it actually reveals a misconception. In a modern GxP environment, Electronic Batch Record (EBR) functionality isn’t something that’s integrated into the Manufacturing Execution System (MES), rather it resides within it as a core module.
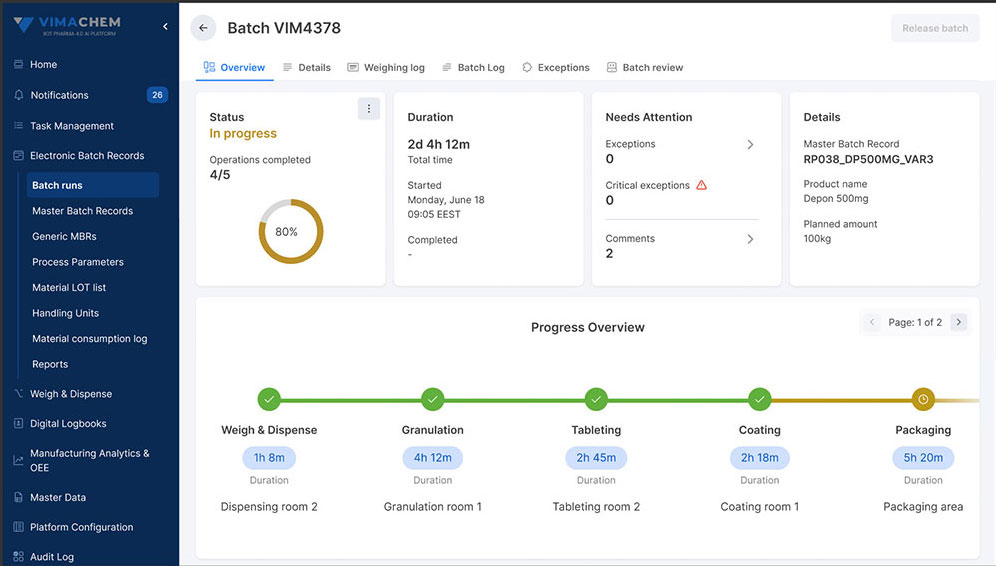
Let’s clarify the difference. An MES is a comprehensive platform that manages production operations in real time, coordinating materials, equipment, personnel, and execution logic across the shop floor. EBR, on the other hand, is a specific application that captures, manages, and secures batch documentation in a GMP-compliant, digital format. In today’s Pharma 4.0 landscape, EBR is not a standalone system requiring integration with your MES, but rather a key module embedded inside a well-architected MES. That’s exactly how Vimachem’s MES is built, with EBR functionality and other core modules, like Manufacturing Analytics & OEE, Weigh and Dispense and many more.
What are the Key Differences:
MES
EBR
Purpose
End-to-end production management
Capture and review batch production data
Scope
Broad – includes scheduling, materials, personnel, equipment, OEE, etc.
Narrow – focused on compliance, documentation, and traceability
Users
Operators, supervisors, planners
QA, production, compliance teams
Regulatory Use
Supports GMP through controls
Critical for GMP documentation and review
MES
Purpose
End-to-end production management
Scope
Broad – includes scheduling, materials, personnel, equipment, OEE, etc.
Users
Operators, supervisors, planners
Regulatory Use
Supports GMP through controls
EBR
Purpose
Capture and review batch production data
Scope
Narrow – focused on compliance, documentation, and traceability
Users
QA, production, compliance teams
Regulatory Use
Critical for GMP documentation and review
To simplify:
- MES is the overall platform, while
- EBR is a key application inside the platform
Why do so many companies still talk about an MES-EBR integration?
So why do so many companies still talk about an MES-EBR integration? In most cases, they’re either working with outdated systems where MES and EBR were delivered separately, or they’re trying to modernize paper batch record processes without disrupting production. The goal is clear. They want to link batch documentation to live process data but the method doesn’t require an integration if you choose a platform designed for it. With Vimachem, batch records, operator actions, sensor data, material movements, and exception reports all flow into a unified, validated system. There’s no need for third-party connectors, custom scripts, or integrations between tools.
Are There Any Drawbacks to Implementing an EBR?
Some pharmaceutical manufacturers are skeptical about moving to EBR mainly because various EBR platforms require considerable time and resources to set up and run with existing systems and processes.
However, with the right software and proper training, introducing a digital batch record solution into your operations is manageable and straightforward.
Indeed, thanks to today’s IIoT-based MES, batch record implementations are smoother than ever!
Is It Worth It?
Within the cohesive operational framework of an MES, electronic batch records become a reliable, real-time source of truth, enhancing accuracy, ensuring compliance, and increasing efficiency across production. This foundation strengthens traceability, streamlines workflows, and reinforces both Quality Control (QC) and overall productivity.
Today, Pharma companies that take smart advantage of all their resources can achieve operational excellence and position themselves for future growth and innovation in a highly competitive industry.
Consider Some Figures On Pharma Manufacturing
The FDA approves an average of 53 new drugs per year. Meanwhile, US life science companies spend over $70 billion on pharma R&D.
Nevertheless, they have to wait 10 to 15 years from the initial discovery of a drug to regulatory approval – provided all clinical trials succeed, that is.
So, out of all those drugs that enter development, a mere 12% get FDA-approved.
Once authorized, ‘blockbuster’ drugs have only a few years to achieve high ROI before their patent expires and competing products enter the market.
Some very tight timelines indeed.
Cloud-based solutions and integrations help optimize processes at all levels, considerably improving time to market.
When EBR is part of your MES Connected Ecosystem...
You are enabled to:
- Access enterprise-wide information.
- Deliver role-based instruction to workers.
- Provide exception-based reporting.
The result being:
- An accelerated time-to-market.
- Reduced costs across multiple areas.
- Simplified, provable, and continuously improving compliance.

EBR within an MES & Automation: A Winning Combination
Thanks to the digital transformation in pharma, manufacturing processes are more automated, and efficient than ever.
Among equipment, people, materials, QC results, and deviations, an IIoT MES collects real-time data from disparate sources and stores it in its ‘track and trace’ database in the cloud.
The electronic batch record system can auto-fill the appropriate data for every batch production and use advanced analytics to identify patterns and improve decision-making.
Such robust collaborations shorten R&D time, improve reporting, and optimize production.
For example, with EBRs, recipe elements can be executed simultaneously by multiple shop floor operators, while, on the production line, recipe tasks can freely move between staff and departments without risking losing data or downgrading execution.
With a cloud-based EBR, collaboration and mobility are enhanced, users can securely access the system from any laptop or mobile device through authenticated login, without compromising data integrity.
More than that, automated reporting capabilities help staff focus on their core responsibilities rather than getting bogged down in reporting batch production events.
In A Nutshell: MES - Electronic Batch Record Benefits
Integrating electronic batch records as part of your MES system helps pharmaceutical companies overcome the challenges of record-keeping in a highly regulated industry.
Let’s recap the key advantages:
Data Integrity & Compliance
Because MES systems are core to GMP (Good Manufacturing Practices) compliance, automating data collection and entry with an EBR application versus a paper batch record minimizes the likelihood of human error, which is often prevalent in manual systems.
This approach ensures that every step of the manufacturing process is accurately documented and traceable, maintaining data integrity and authenticity.
Traceability & Transparency
Having an EBR deployed within your MES allows the real-time capture of all relevant data, from raw materials to finished product and it assures detailed and transparent traceability and facilitates auditing.
Quality Assurance & Quality Control
This approach enforces quality checks throughout every production stage, allowing for immediate deviation detection and correction.
Continuous monitoring of Critical Quality Attributes (CQAs) and Critical Process Parameters (CPPs) guarantees consistent product quality and compliance.
Cost Optimization & ROI
There are significant cost savings, and these can be quickly realized when you partner with providers like Vimachem who enable fast implementations and operate a “no hidden cost” policy.
The automated data collection and reporting optimizes processes and can significantly reduce costs, resulting in shorter batch release times and a greater return on investment (ROI).
See how Vimachem's EBR module can streamline production, reduce errors, and improve regulatory compliance.
Elevate Pharma/Biopharma Production With Vimachem’s MES-EBR Solutions
If you’re searching for MES-EBR integration, what you really need is an MES with embedded EBR capabilities, like Vimachem and Vimachem’s Electronic Batch Record system is specifically designed for the Life Science industry.
Considering the ever-changing compliance regulations, the Vimachem cloud-based EBR solution easily adapts to new processes and products and scales seamlessly as your business grows.
It can be standalone or integrate smoothly with other modules within the Vimachem MES platform and your IoT devices, providing real-time feedback and secure cloud storage for indisputable data quality. To find out more, schedule a demo with a member of the Vimachem team.