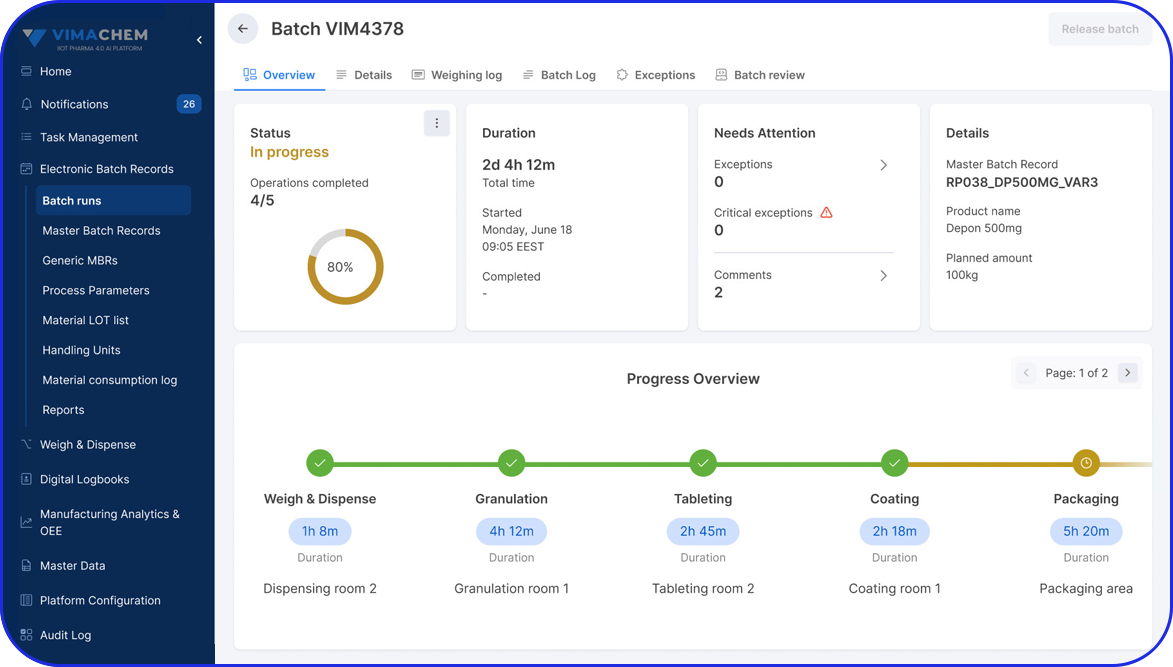
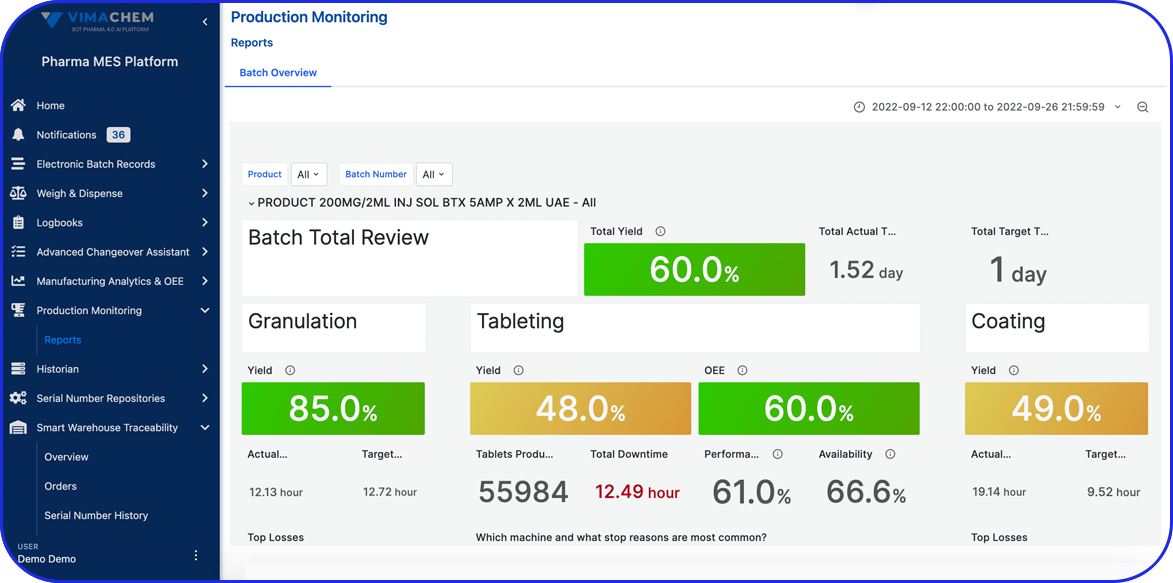
Weigh & Dispense Module
A digital solution that enables you to streamline your dispensing activities and ensure compliance & efficiency. Built for Pharma & Biopharma manufacturers, the software is pre-validated & built on modern technology, enabling faster and more robust implementations.

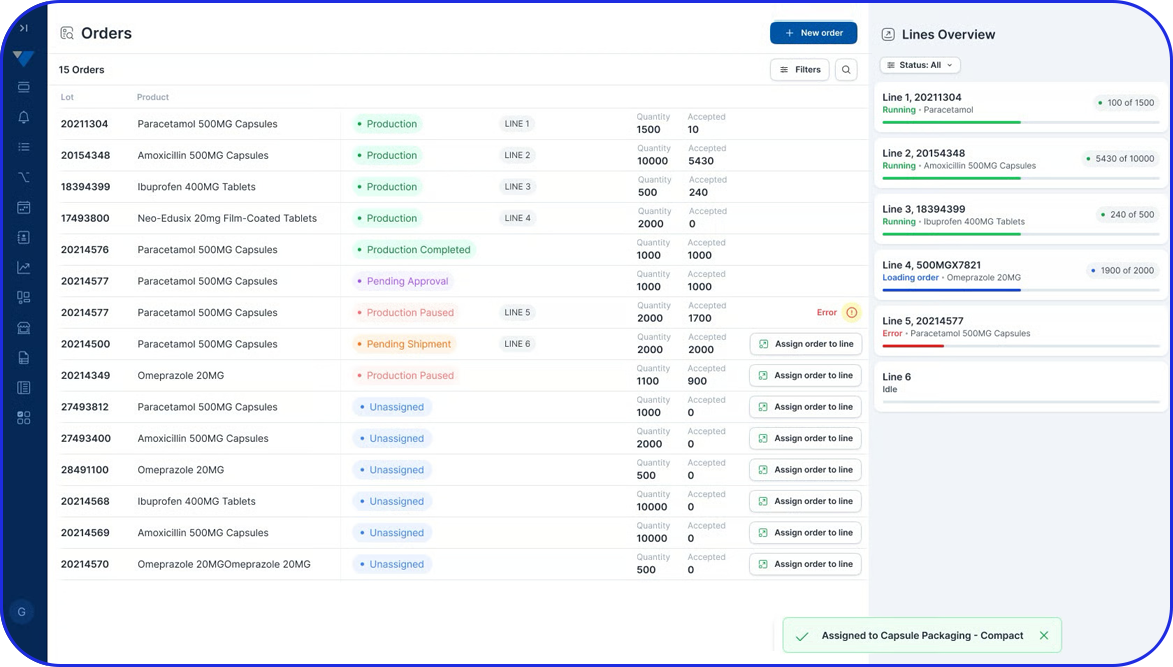
Streamline your Weighing & Dispensing Operations
Streamline your Weighing & Dispensing Operations
Time and Cost Savings
The system automates weighing and dispensing processes, reducing manual labor and minimizing the time required for each operation. These benefits lead to increased efficiency, shorter production cycles, and potential cost savings.
Enhanced Safety and Operator Guidance
The software system includes safety features and operator guidance, such as handling instructions, safety precautions, and warnings for hazardous materials. This promotes a safe working environment, reduces the risk of accidents, and ensures operator compliance with safety protocols.
Integration and Data Exchange
The system integrates with other enterprise systems, such as Manufacturing Execution Systems (MES) and Enterprise Resource Planning (ERP), facilitating seamless data exchange and end-to-end visibility across the manufacturing operations.
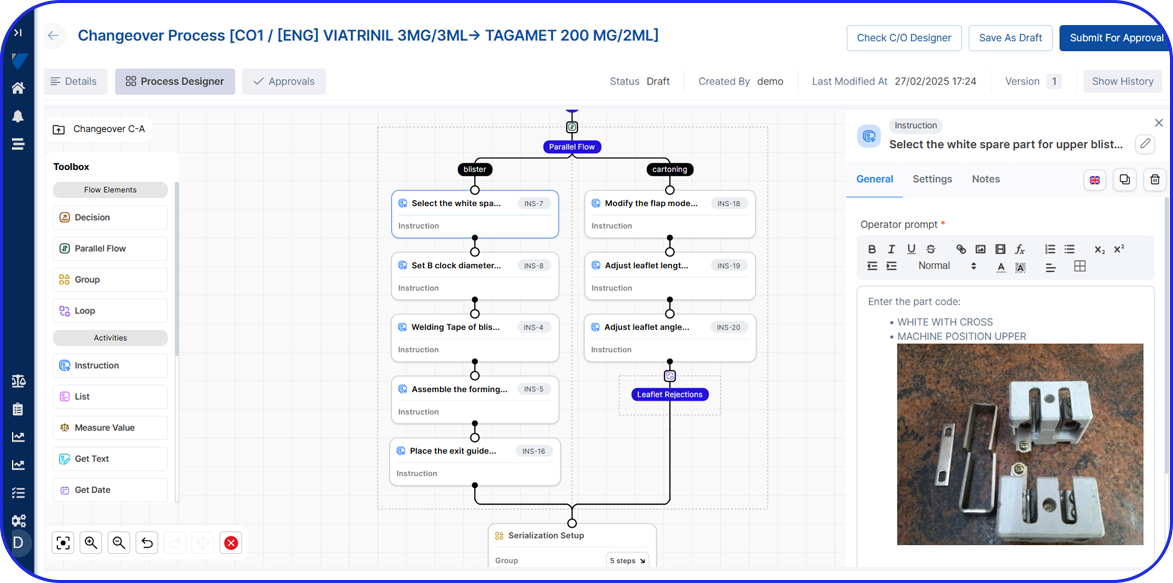
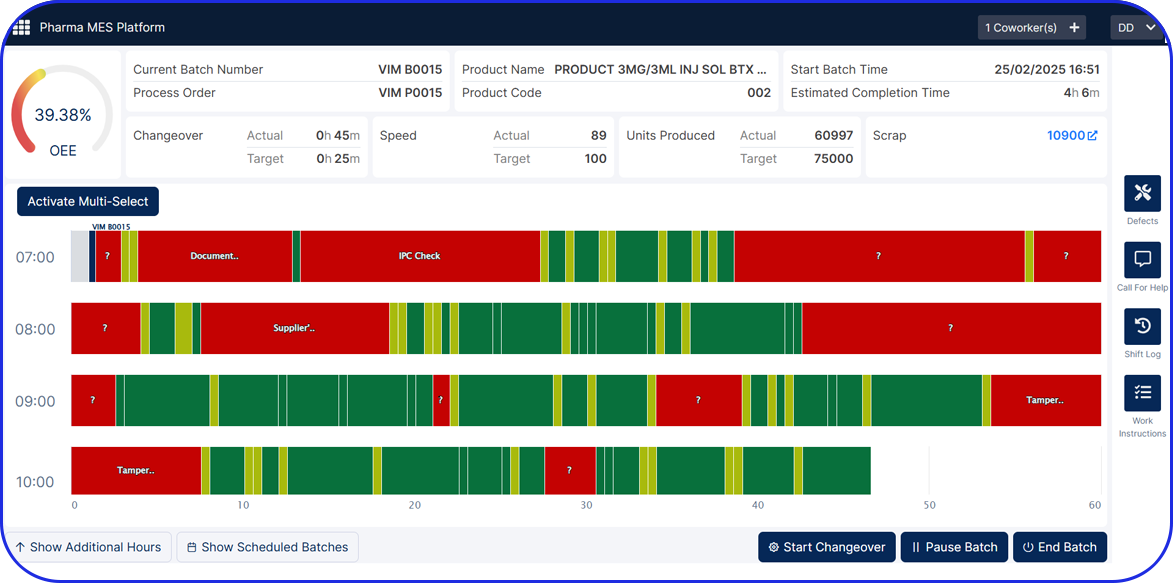
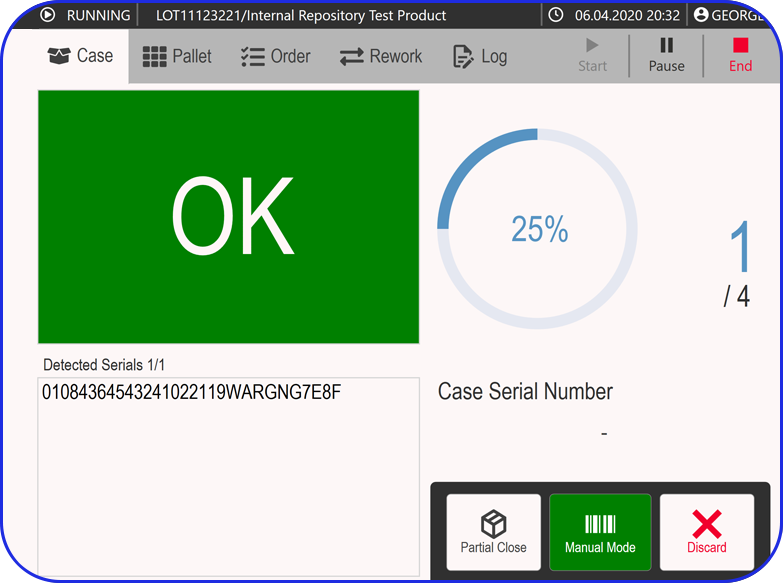
See Vimachem’s WDM in Action
Benefits of Vimachem's Weigh and Dispense Software
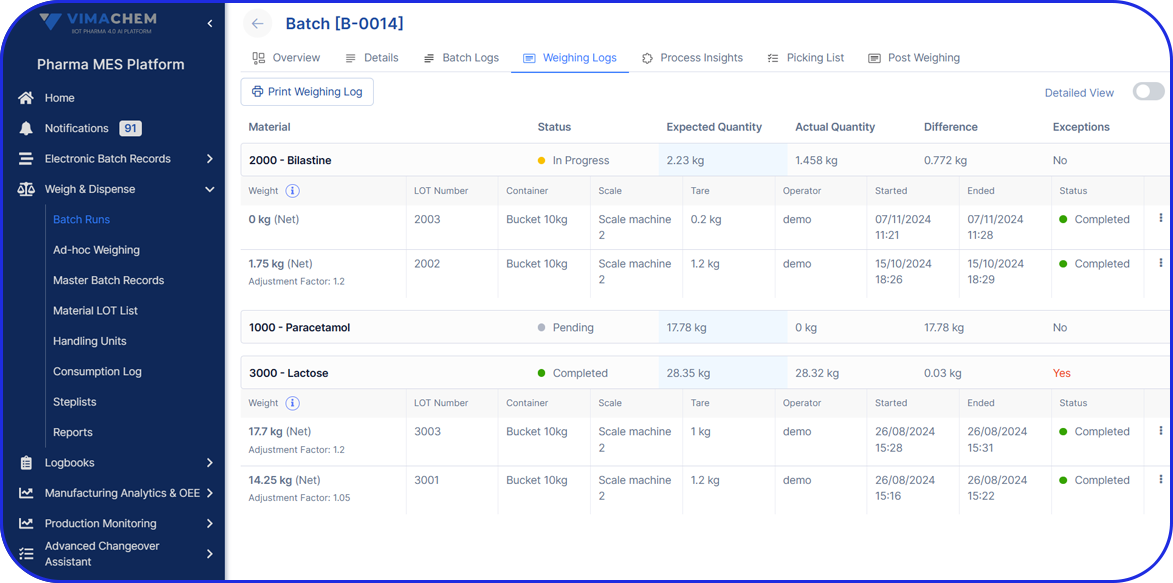
Accuracy and Precision
The software system ensures accurate and precise weighing and dispensing of raw materials, reducing the risk of errors and improving batch quality and consistency. This helps maintain product integrity and compliance with regulatory requirements.
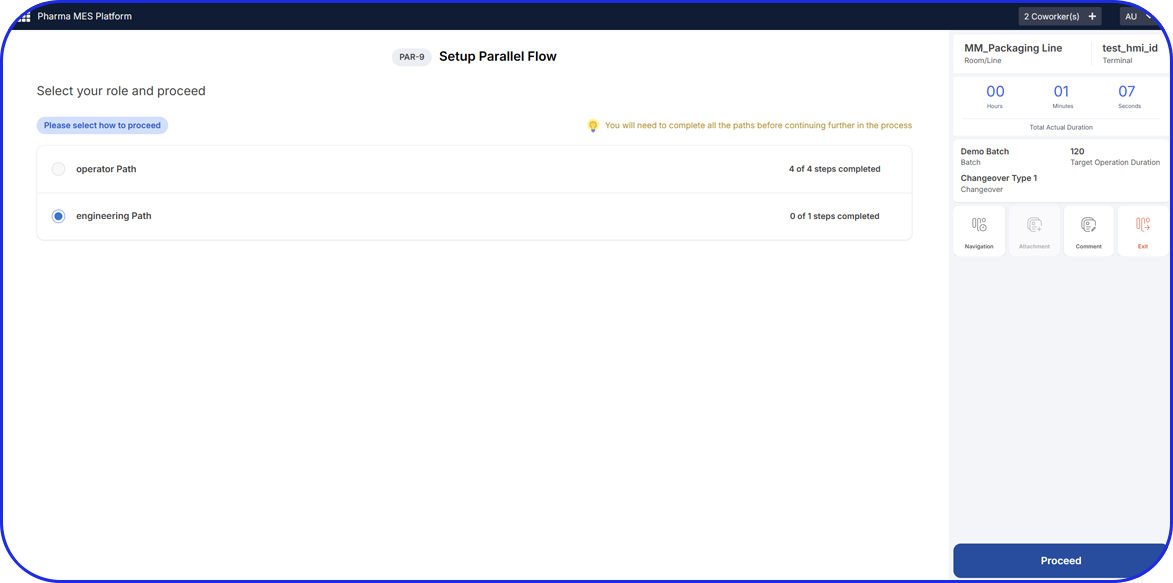
Improved Workflow and Efficiency
The software system streamlines the weighing and dispensing workflow, providing step-by-step guidance to operators. It optimizes the sequence of operations, reduces process variability, and eliminates guesswork, resulting in improved overall efficiency and productivity.
Real-time Inventory Management
The system integrates with inventory management systems, allowing real-time tracking of raw material quantities and ensuring accurate inventory levels. This helps prevent stockouts, reduces waste, and enables effective inventory planning and control.
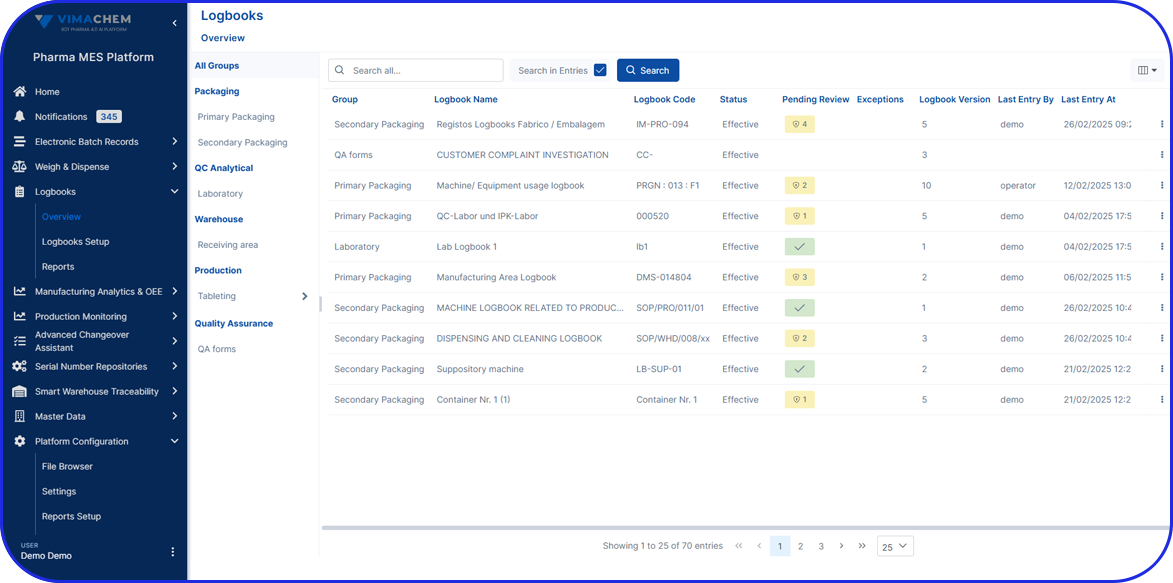
Compliance and Traceability
The software system enforces compliance with regulatory standards, such as Good Manufacturing Practices (GMP). It maintains detailed electronic records, including batch information, operator details, timestamps, and material traceability, facilitating audits, investigations, and regulatory reporting.
Error Prevention and Quality Assurance
The system incorporates built-in checks, such as barcode scanning and RFID verification, to prevent mix-ups and ensure correct material selection. It also includes tolerance checks, alerts for out-of-specification conditions, and quality control measures to improve overall product quality and reduce deviations.
Documentation and Reporting
The software system generates accurate and comprehensive documentation, including labels and reports, with electronic signatures and timestamps. This simplifies documentation processes, reduces paperwork, and supports efficient record-keeping and compliance with documentation requirements.
Embed Quality into Your Weigh and Dispense Process for Pharma Compliance
GxP Compliance in Weigh and Dispense Systems
User-Level Permissions & Security
Manage roles and permissions to keep data safe. User administration in a Windows Active Directory simplifies the definition of password criteria, reset and change periods, and reduces IT support demands.
Data Integrity
The system is verified for data integrity, with advanced controls that allow verification of inputs during the operation and controlled access with role-based access management.
Electronic Records & Signatures
Fully compliant with FDA Title 21 CFR Part 11 and EU GMP Annex 11 (Electronic Records and Signatures).
Digital Records
The system holds digital records of every operation on the shop floor, including device records and equipment logs.
Electronic Signatures
Efficient review and approval through configurable workflows ensure quality remains consistent during the production process.
Data Exports
All data can be exported manually and automatically in Microsoft® Excel® (XLSX) and Extended Markup Language (XML) formats.
FAQs on Weigh & Dispense
What is weigh and dispense in pharma?
Weigh and dispense refers to the process of accurately measuring raw materials and dispensing them into containers for batch production. In pharma, this step is critical for ensuring product quality, safety, and compliance with GMP requirements.
What is the difference between weighing and dispensing?
Weighing is the process of accurately measuring the required quantity of raw materials. Dispensing is the transfer of those weighed materials into the correct container or process step. Together, they ensure batches are prepared precisely and consistently.
What is the difference between distributed and dispensed?
“Dispensed” typically refers to the preparation and delivery of medicines or raw materials for immediate use, while “distributed” describes the larger-scale movement of products through supply chains, warehouses, or markets.
What is weighing in pharmacy?
Weighing in pharmacy is the accurate measurement of ingredients used in drug formulation. It ensures that each batch contains the exact quantities required, preventing quality deviations and ensuring compliance with regulations.
Why is accuracy so important in weigh and dispense?
Small errors in weighing or dispensing raw materials can compromise batch quality, cause deviations, and even trigger regulatory non-compliance. Accurate systems help maintain product integrity and patient safety.
How does a digital Weigh & Dispense Module improve compliance?
Digital WDM systems enforce GMP guidelines, maintain electronic records and signatures, and provide full traceability of every operation. This simplifies audits and supports compliance with FDA and EU regulations.
Can the Weigh & Dispense Module integrate with other systems?
Yes. The system integrates with MES and ERP platforms, enabling seamless data exchange, real-time inventory updates, and end-to-end visibility across the manufacturing process.
What role does barcode or RFID scanning play in weigh and dispense?
Barcode and RFID checks prevent mix-ups by verifying raw materials before weighing. They also support traceability, reduce errors, and add an extra layer of quality assurance.
How does Weigh & Dispense Module support data integrity?
The module enforces role-based access, electronic signatures, and audit trails. Every input is verified, and all operations are recorded digitally, ensuring compliance with FDA 21 CFR Part 11 and EU GMP Annex 11.
How does the Weigh & Dispense Module improve batch traceability?
The module records every step of the process from raw material identification to operator actions and timestamps. This ensures complete electronic traceability, making audits easier and strengthening compliance with GMP standards.
Contact us
Ready to Augment your Shop Floor Operations?
Ready to Augment your Shop Floor Operations?
Get started with real-time manufacturing analytics today.