Manual Pharmaceutical Aggregation Software
Manually aggregate up to three subsequent packaging levels and comply with worldwide pharmaceutical regulations in a perfect solution for manual, low-speed lines.
See Vimachem’s MAM in Action
What is the Manual Aggregation Module (MAM)?
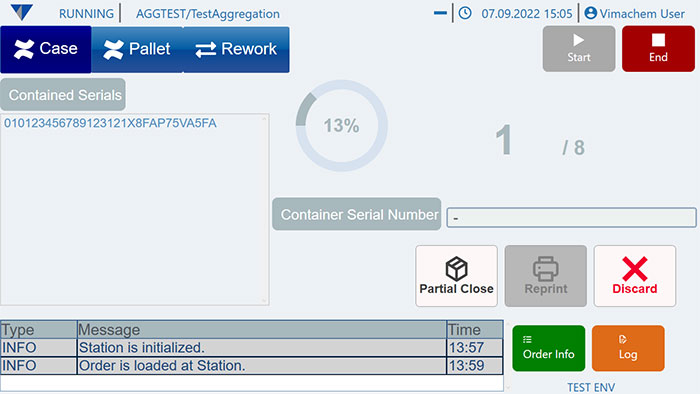
The Vimachem Manual Aggregation Module (MAM) is a manual pharmaceutical aggregation software designed to streamline aggregation in pharmaceutical products. As a pharma aggregation solution, MAM enables the manual aggregation in pharma by linking individual product packages to higher packaging levels, ensuring full traceability.
As part of Vimachem’s open serialization platform, this aggregation software integrates seamlessly with various IT systems and third-party equipment. The MAM allows manual aggregation of up to three subsequent packaging levels, helping pharmaceutical companies comply with global regulations. It is the ideal aggregation solution for manual, low-speed production lines, ensuring regulatory compliance and operational efficiency.
Benefits of Manual Pharmaceutical Aggregation Software

Aggregation made easy
User friendly interface and automated interfaces to industrial printers (e.g. Zebra) and scanners (Zebra, Datalogic etc.).

Time saving
Quick product returns and recalls. The capacity to effectively manage the entire inventory return process is made possible through aggregation technology.

Handle post batch rework
Handle sampling, verification, or damaged product without having to send product back to the packaging site and line.

Aggregation compliance
Enables compliance with markets (RU, UZB, KAZ etc.) that require aggregation.
Frequently Asked Questions
What is aggregation in pharma?
Pharmaceutical aggregation refers to the process of linking individual pharmaceutical products to higher packaging levels (such as bundles, cases, or pallets) for traceability and compliance. It’s a critical step in the pharmaceutical supply chain, ensuring that products can be tracked from the smallest package to the final shipment. This process helps prevent counterfeiting and supports regulatory compliance in the pharma aggregation process.
How does pharmaceutical aggregation work?
Pharmaceutical aggregation works by assigning unique identifiers to individual packaging levels and linking them together. Using aggregation software, this data is then consolidated into higher packaging levels, ensuring complete traceability across the entire supply chain. The system captures and stores information such as serial numbers, batch numbers, and expiration dates, making it easy to track the product's journey from manufacturing to distribution. For low-speed lines, manual aggregation can be used to aggregate up to three packaging levels efficiently.
What is the difference between aggregation and serialization?
While serialization refers to assigning a unique serial number to each individual product unit, aggregation involves linking these serialized units to higher levels of packaging. In other words, aggregation in pharmaceutical products groups serial numbers from individual units into higher-level packages (e.g., boxes or pallets). Both processes are essential in ensuring the traceability and authenticity of pharma aggregation, but serialization focuses on individual units, while aggregation ensures complete packaging traceability.
Ready to Augment your Shop Floor Operations?
Ready to Augment your Shop Floor Operations?
Get started with real-time manufacturing analytics today.










