Product Tour
Accelerating Digital Transformation in Pharma
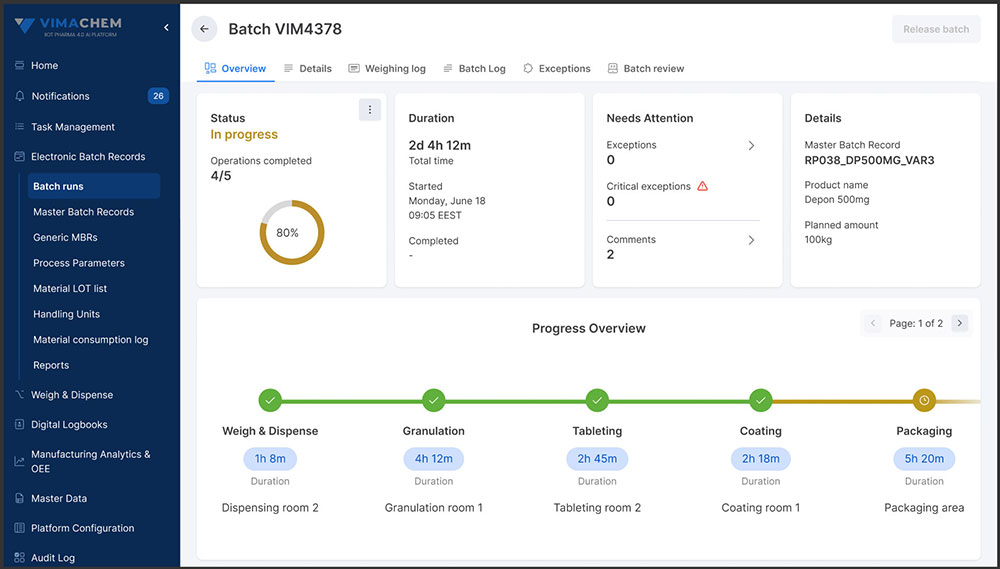
Faster, Smarter Batch Reviews
Manual batch reviews can be time-consuming and resource-intensive, often delaying product release. Vimachem’s review-by-exception approach surfaces critical deviations, allowing quality teams to focus their expertise where it’s needed most - accelerating approvals while upholding compliance and product integrity
Pre-Validated for Rapid Deployment
Implementation delays due to lengthy validation cycles can stall digital transformation and increase costs. Vimachem’s pre-validated solution includes a comprehensive, out-of-the-box validation package -reducing deployment timelines by at least two months while ensuring full compliance from the initial rollout.
Seamless System Integration
Disruptive system overhauls and poor interoperability can slow adoption and increase risk. Vimachem’s solution is built to integrate effortlessly with your existing ERP, MES, QMS, and lab systems -minimizing setup time and ensuring a smooth transition without added infrastructure investment.
See Vimachem’s eBR in Action
Built for Manufacturing, Quality, and Compliance Teams in Pharma & Biopharma
Pharmaceutical and biopharmaceutical manufacturers face increasing pressure to ensure batch integrity, maintain compliance, and improve operational efficiency, all while reducing manual effort and review time. Vimachem’s Electronic Batch Records (eBR) solution addresses these challenges through automation, standardization, and real-time visibility – accelerating batch release and supporting regulatory compliance with minimal disruption.
Vimachem’s eBR solution empowers manufacturing and quality teams to digitize operations, maintain compliance, and accelerate batch release – delivering faster, smarter, and more resilient manufacturing across all sites.
eBR provides:
- A pre-validated solution with a complete IQ/OQ package, significantly reducing implementation time.
- Built-in compliance with FDA, GMP, and 21 CFR Part 11 for secure, validated, and auditable data and processes.
- A flexible framework that adapts easily to product changes, regulatory updates, and evolving processes.
- Seamless integration with IoT devices and smart sensors to improve data accuracy and enable real-time monitoring.
- Scalable architecture that supports multi-site operations and global manufacturing needs.
- Minimal training requirements thanks to an intuitive interface and streamlined workflows.
Accelerate Time to Market with Smart, Compliant, and Flexible eBR
Automated Data Capture & Real-Time Visibility
Eliminate manual data entry with automated, real-time tracking of production activities - enhancing accuracy, reducing delays, and enabling data-driven decisions across your operations.
Faster, Right-First-Time Batch Reviews
Reduce QA review time through automated checks, exception handling, and right-first-time execution - minimizing rework, improving compliance, and speeding up batch release.
Configurable Workflows & Role-Based Access
Tailor workflows, approvals, and user permissions to match your specific processes - ensuring compliance with regulatory requirements while improving operational agility.
Advanced Reporting & Operational Dashboards
Generate detailed reports and visualize production trends with built-in dashboards - supporting continuous improvement, faster audits, and informed decision-making.
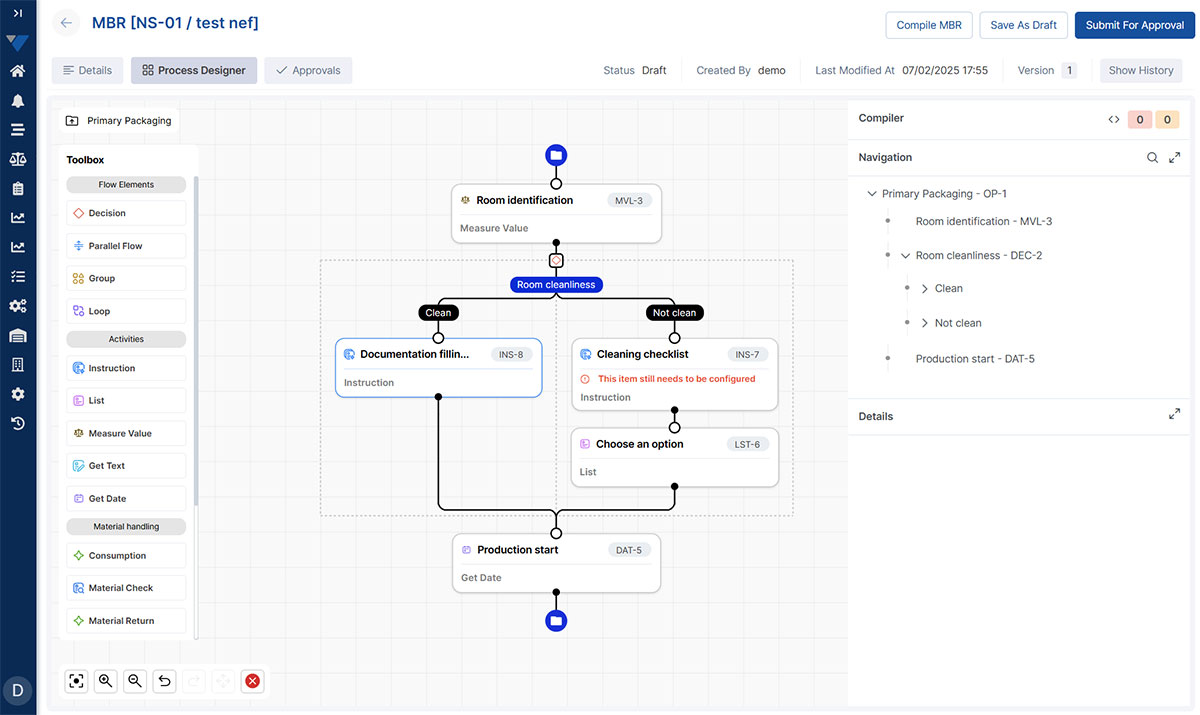
Scalable Recipe & Process Management
Adapt easily to new products, regulatory updates, or process changes with a flexible, low-maintenance recipe framework designed to grow with your operations.
Mobile-Friendly & Device
Agnostic
Access and manage production data securely from any device, anywhere - enabling real-time collaboration and responsiveness across teams and manufacturing sites.
Vimachem’s eBR has transformed our batch release, cutting review time by half while ensuring full regulatory compliance.
VP of Quality Operations, Global Pharmaceutical Manufacturing organization
GxP Features
User-Level Permissions & Security
Manage access with role-based permissions and Active Directory integration - enabling secure login, password policies, and simplified user administration with reduced IT overhead.
Verified Data Integrity
Ensure compliant, accurate data with built-in input validations, audit trails, and restricted access based on user roles - supporting data integrity at every stage of production.
Electronic Records & Signatures
Compliant with FDA 21 CFR Part 11 and EU GMP Annex 11, the system ensures secure, traceable, and auditable records with verified electronic signatures.
Complete Digital Records
Capture a full digital history of all shop floor activities, including device usage, material handling, and equipment logs - ensuring transparency and traceability.
Configurable Electronic Signatures
Enable efficient review and approval with electronic signatures embedded in workflow steps - ensuring compliance and consistency across all production stages.
Flexible Data Exports
Easily export data in XLSX and XML formats, manually or automatically - simplifying reporting, analysis, and system integrations.
Scalable Expansion with Modular Add-Ons
Vimachem’s eBR goes far beyond batch records – you can seamlessly integrate Manufacturing Analytics & OEE, and Weigh & Dispense to gain complete process visibility, operational excellence, and compliance across every stage of production.
Weigh & Dispense
Manufacturing Analytics & OEE
Contact us
Ready to Augment your Shop Floor Operations?
Ready to Augment your Shop Floor Operations?
Get started with real-time manufacturing analytics today.