Digital Forms & eLogbooks for Pharma
Easily design any form and logbook, maximize data integrity, and enable real-time monitoring of all production, cleaning, maintenance, breakdowns, or calibration events.

Eliminate Paper & Reduce Costs
Avoid mistakes and reduce the time and costs of filling in and handling paper records by making it easy for everyone to access and read maintenance/quality forms and logbooks.
Error-Proof Auditable Secure Data Storage
Replace old paper logbooks and forms, meet FDA and European Commission documentation requirements, comply with FDA CFR 21, Part 11, and maximize data integrity.
Real-Time Visibility & Analytics
Enable visibility into plant equipment even if away from the shop floor with real-time access to process and maintenance events and advanced analytics on historical data and KPIs.
Optimize Pharma Operations with Paperless Documentation & Real-Time Insights
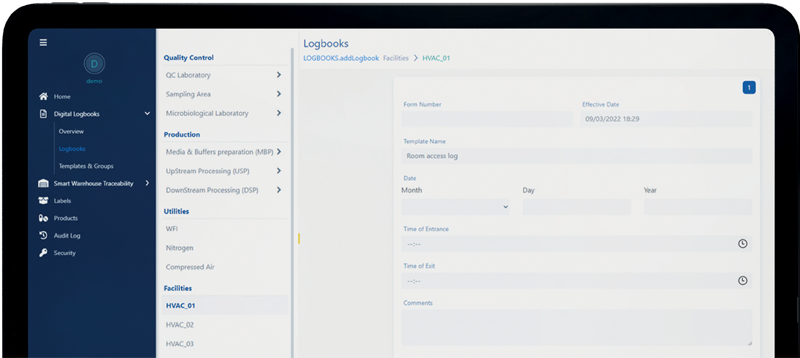
What is the digital forms and eLogbooks module?
The Vimachem Digital Forms and eLogbooks module is a cloud-based electronic logbook software designed for pharmaceutical and biotech sites. It enables seamless digitization of paper-based logbooks and forms within minutes while ensuring full compliance with Current Good Manufacturing Practice (CGMP).
The electronic logbook allows for real-time data collection and approval through a user-friendly web interface, enhancing audit readiness via its authentication and blockchain capabilities. As a trusted solution among leading Pharma and Biotech companies, Digital Forms and eLogbooks Module provides secure data access, instant insights, and improved operational efficiency.
With digital forms and electonic logbook software, companies can save time, reduce costs, and enhance data integrity while ensuring compliance with industry regulations.
Benefits of Digital Forms & eLogbooks
Paperless Documentation
Paper logbooks and forms make validation difficult, leading to financial loss, human error, and work redundancy. Delays in detecting deviations impact compliance and production efficiency, while inaccurate estimates of production quantities result in waste and loss of customer confidence.
Digitization of Pharma Operations
The digitization of logbooks, forms, and end-of-day processes - including readings, observations, alerts, and messages - ensures right-first-time execution. This improves GMP compliance, enables review by exception, and reduces manual effort, increasing overall operational efficiency.
Enhance Security & Scalability
Electronic logbooks provide secure data access through trusted network devices, ensuring data integrity and compliance. The technology is fully scalable, growing alongside your pharma plant operations to meet evolving industry needs.
Instant Access and Full Transparency Across Devices

Features of Vimachem's Digital Forms & eLogbooks App for Pharma
Pre-Packaged for Speed
A disruptive app, pre-packaged to expedite implementation time and accelerate ROI realization.
Reduces Total Cost of Ownership
Engineered to significantly cut TCO – by up to 80% – through streamlined deployment and maintenance.
Fully Scalable Technology
Uses scalable infrastructure that grows with the business and adapts to evolving manufacturing needs.
Real-Time Manufacturing Oversight
Provides a continuous, real-time view of the entire manufacturing process from a single interface.
Automatic Exception Reporting
Tracks and flags deviations automatically, enabling faster response with minimal manual effort.
Why Choose Vimachem’s Electronic Forms & Logbooks Module?

Go paperless
No more paper in circulation and for filing.

Generate insights
See what the issues were during difficult production runs?

Avoid mistakes
Get instant access to the logbook /form where all issues are recorded.

Improve efficiency
Save time, improve data exchange and communicate easily.

Traceability
Quickly trace issues on shifts in the production records.

Save costs
Cut the costs of filing and handling paper records and enable review by exception.
Frequently Asked Questions
What is a digital form?
A digital form is an electronic version of a traditional paper form used for capturing, storing, and processing data. In pharma and biotech industries, digital forms replace manual data entry with automated, structured input fields, ensuring real-time validation, compliance, and traceability.
What is an electronic logbook?
An electronic logbook (e-logbook) is a digital alternative to traditional logbooks, designed to track manufacturing, quality, and compliance-related activities in regulated industries. Unlike paper-based logbooks, electronic logbooks provide secure, timestamped entries, real-time data access, and automated audit trails.
How do electronic logbooks work?
Electronic logbooks work by digitizing manual record-keeping processes, ensuring accuracy, security, and efficiency. They replace handwritten entries with structured digital inputs, reducing the risk of errors, missing data, and unauthorized modifications.
How do you use an electronic logbook?
Using an electronic logbook involves following a streamlined process designed to improve data integrity, accessibility, and compliance.
How to Use an eLogbook Effectively:
• Choose the right logbook for your operation (e.g., batch processing, equipment log, safety checklist).
• Input data digitally using structured forms with automated field validation.
• Access logs remotely from any authorized device, ensuring real-time visibility.
• Review & approve entries using digital signatures for GMP compliance.
• Generate reports & insights with built-in analytics tools.
What are the use cases of electronic logbooks and digital forms in Pharma & Biotech?
Electronic logbooks and digital forms play a critical role in pharmaceutical manufacturing and biotech operations, ensuring compliance, efficiency, and traceability across different processes.
Key Use Cases:
• Batch Record Documentation - Automate data entry for batch manufacturing records, ensuring real-time review and GMP compliance.
• Equipment Maintenance Logs - Digitally track calibration, servicing, and breakdowns to improve asset management and uptime.
• Deviation & CAPA Management - Record deviations, assign corrective actions, and track resolution timelines with full audit trails.
• Environmental Monitoring - Capture real-time temperature, humidity, and contamination data to meet regulatory requirements.
• Operator Shift Logs - Maintain structured logs of shift activities, handovers, and process changes.
• Audit & Inspection Readiness - Store secure, timestamped records that simplify regulatory audits and compliance reviews.
Ready to Augment your Shop Floor Operations?
Ready to Augment your Shop Floor Operations?
Get started with real-time manufacturing analytics today.










