Computerized System Validation
We specialize in Computerized System Validation (CSV) and Quality Assurance (QA) support services to our global customers in the life-sciences industry.
We provide computerized system validation (CSV) services
to life sciences companies throughout Europe that manufacture products for human use/consumption.
We ensure that our customers’ computerized systems holding GxP data are validated
based on GAMP5 guidelines, so that they meet user, technical and regulatory requirements (e.g. FDA, MHRA, ICH, ISO regulations). GxP data are critical data obtained during product manufacturing & distribution and laboratory testing that are mostly included in product batch records and therefore susceptible to inspections by regulatory authorities.GxP = Good [X: Automated Manufacturing, Laboratory, Distribution] Practices.
We support organizations belonging in the life sciences industry
that produce pharmaceutical products, medical devices, dietary supplements, vitamins, cosmetics, veterinary products and tobacco products to meet the required and applicable compliance requirements for their computerized systems.
We also provide:
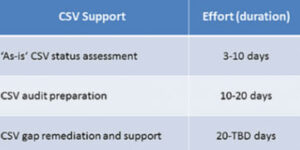
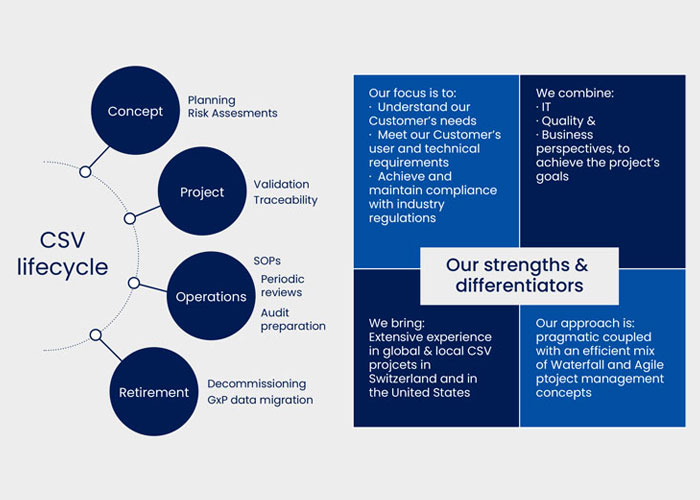
- Quality Assurance support (QA) support throughout the entire CSV lifecycle (Concept, Validation/Project, Operation, Retirement)
- Change management, CAPA management, periodic reviews
- Creation and optimization of our customer’s Quality Management System (QMS)
- Creation of Standard Operation Procedures (SOPs)
Why choose Vimachem as your CSV and QA support partner:

We have over 15 years experience
in the United States and in Europe in the field of Quality Management and Computerized System Validation.

We are fully committed
to creating value for our customers.

Our model is to provide csv as a service (CSVAAS) support
where our customers choose one or more of our services according to their priorities and needs.
We provide the required solution packages
to our customers.
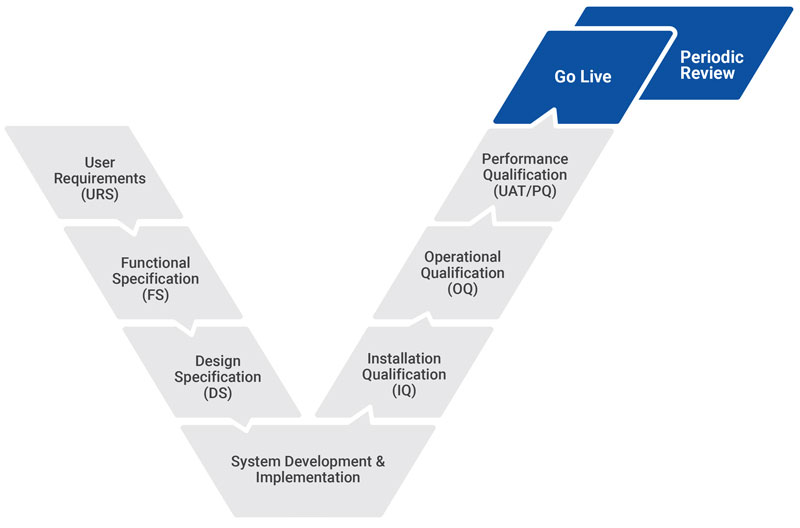
Specifically our CSV experts provide:
Specifically our CSV experts provide:
- System risk assessments (ISRA, FMEA, FRA)
- Computerized System classifications
- Collection of requirements and identification of specifications (URS, FS, DS CS)
- Validation project planning and execution (VP, Test Cases, Test Report)
- Traceability matrix and validation reports (TM, VR)
Quality Management Systems (QMS), Laboratory Information Management Systems (LIMS), Enterprise Resource Planning systems (ERP), Manufacturing Execution Systems (MES), Electronic Laboratory Notebooks (ELN), Laboratory data acquisition software, Programmable Logic Controllers (PLCs) and other laboratory and manufacturing electronic systems that need to comply with industry guidelines (FDA 21 CFR Part 11, EU Annex 11, GAMP5.
Examples of computerized systems we validate
Examples of computerized systems we validate
Ready to Augment your Shop Floor Operations?
Ready to Augment your Shop Floor Operations?
Get started with real-time manufacturing analytics today.