Digital-By-Design Pharma: Why the Next Era of Pharma Manufacturing Requires MES as Core CAPEX - not a Side Project
Digital-By-Design Pharma: Why the Next Era of Pharma Manufacturing Requires MES as Core CAPEX - not a Side Project

Emma Hanley
Pharma Digital Transformation Consultant
Reflections from the ISPE Ireland Annual Conference 2025 -"Pioneering Tomorrow: The Convergence of Innovation & Life Sciences"
The 2025 ISPE Ireland Annual Conference underscored a truth that the industry has been circling for years but is now impossible to ignore: Digital execution is no longer a follow-on initiative. It must be built into the facility, process, and operating model from day zero.
For more than a decade, the Life Sciences sector has spoken confidently about digital transformation. Every keynote, every strategy deck, every steering committee has repeated the same promises: smarter factories, real-time data, predictive quality, autonomous operations.
Yet across the industry, the reality on the ground remains uneven. Some organizations from today’s presentations e.g. Takeda, Lilly, J&J, are proving what digital-first manufacturing looks like. Many others are still balancing legacy infrastructure, hybrid processes, and the operational realities of facilities that were never designed with digital execution in mind.
The message from the Irish Life Sciences Summit was clear: The industry is entering a phase where competitive advantage will depend on designing, building, and scaling facilities with digital as a foundational layer, while still ensuring that existing plants can retrofit intelligently, efficiently, and modularly.
This future is not “digital-only CAPEX.” It is not “forget the brownfields.” It is the opposite:
Digital maturity now requires two parallel capabilities, digital-by-design for new facilities and modular digital retrofits for existing ones as MES is a strategic, operational and capital decision. And this is where modern MES becomes pivotal as s modular MES allows both worlds to converge:
- Designed into greenfields from concept.
- Retrofitted into brownfields without disruption.
- Scaled line-by-line, site-by-site.
- Standardized globally without over-customization.
This is the architecture the industry has been missing. And the architecture the industry is now demanding because digital-by-design facilities outperform digital-after-the-fact facilities in reliability, speed, quality and cost. But along with technology, what was particularly evident is the human factor – and the ethos that change management and organizational alignment significantly determine the success of any digitalization project.
What follows distils the core messages that emerged across all sessions, highlighting the shifts now defining modern pharma/biopharma operations.
1. The next era of pharma/biopharma requires end-to-end digitalization, not digital afterthoughts
The industry is no longer dealing with single-modality sites or linear recipes. Pipeline expansion, modality diversification, and operational variability require precision, traceability, and adaptive workflows that manual, paper-heavy systems simply cannot deliver.
Discussions highlighted a consistent reality:
The process, the material genealogy, the environmental controls, and the operator interactions are now too complex to layer digital “later.”
When digital is absent at design, teams spend the years compensating for foundational gaps with workarounds, investigations, inefficiencies, and late-cycle tech debt.
When digital is present from day zero, sites gain:
- Structured material flow.
- Automated decision support.
- Data integrity by design.
- Operator-ready workflows.
- A platform for continuous optimization.
This is no longer an efficiency argument. It is a viability argument. It gives you the competitive advantage.
But, while the industry is accelerating toward fully digital-by-design facilities, the reality remains that most of the world’s pharma and biopharma capacity is already built, validated, and deeply productive. These sites cannot wait for a major capital cycle or a ground-up rebuild before they modernize.
This is why modular, configuration-driven MES architectures are not a convenience — they are an operational necessity.
A modern MES must be capable of:
- Quickly deploying into complex, highly validated environments without destabilizing supply.
- Digitizing production workflows in controlled, low-risk phases instead of monolithic “go-lives.”
- Integrating seamlessly with existing equipment, systems, and site constraints.
- Preserving the integrity of ongoing operations while still lifting data visibility, material control, and compliance maturity.
- Have a system that is easy for all users to adopt
This incremental model is not a compromise. It is the only viable modernization path for the global installed base, the facilities that carry today’s biologics, vaccines, and cell and gene therapies.
In other words:
- Digital-by-design must guide new capital projects.
- Modular, frictionless digital uplift must guide the existing network.
A next-generation MES must therefore serve both missions simultaneously:
- Enable full digitalization from day zero for new, high-throughput, multi-modality facilities.
- Modernize the global legacy network without downtime, revalidation shock, or operational risk.
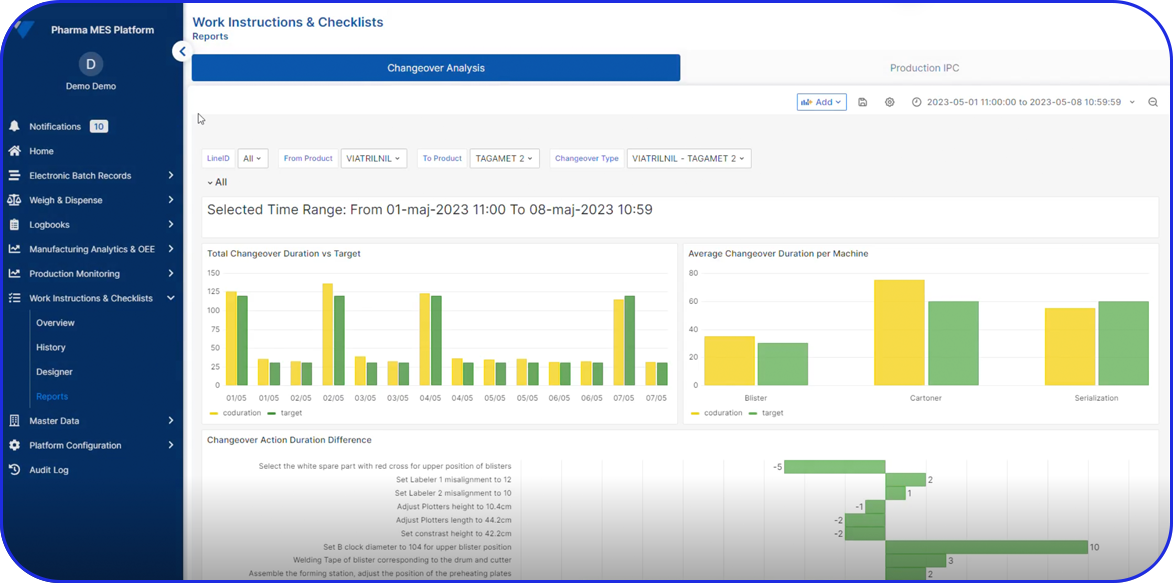
And as Ken Raggett of J&J demonstrated, where their campaign cadence increased from 8 to 12 per year through focused improvements in downtime, changeovers, and data-driven operational readiness – digital uplift directly translates into competitive advantage and significant revenue growth.
2. Digital maturity is maximized when MES is built into the CAPEX blueprint for new sites
An insight that surfaced from the discussions was that for new facilities, true digital maturity can begin when MES is considered from the first stages of capital planning. This is not because retrofits are unworkable. They are both viable and essential for the global installed base.
But new sites operate under a very different economic and operational reality. Capital projects move through fixed stage-gates, fixed scopes, fixed budgets, and fixed validation plans. When MES is designed in from the beginning, it influences far more than software selection, it shapes how the facility will operate, scale, and respond to future modalities.
Early MES integration enables:
- Process and digital design to evolve together, creating a manufacturing model that is executable, efficient, and resilient from day one.
- A unified automation and data architecture, rather than separate layers that must later be harmonized.
- Cleaner validation and qualification pathways, because digital expectations are embedded into the engineering workflow, not retrofitted onto it.
- A single, consistent operational model, reducing variability between equipment, lines, and suites as the site expands.
- Faster path to operational readiness, since digital workflows, material models, and data structures are established before the first batch.
In this model, MES is not an add-on. It functions as part of the facility’s infrastructure, defining how materials move, how decisions are made, how quality is assured, and how data flows across the site and how efficient the overall process is as a whole.
This is why more project teams are treating MES as a foundational element of capital design: it creates alignment across engineering, automation, process design, quality, construction, and operations, and it ensures the new facility opens its doors with digital maturity already built in.
3. Engineering advancements reinforce the need for early digital alignment
The engineering innovations showcased, from mixed-reality facility modelling to autonomous robotic scanning, revealed how rapidly facility design is evolving.
Tools that allow stakeholders to simulate layouts, visualize flows, and identify constructability or operability issues before a single pipe is installed are now mainstream.
One moment captured this vividly: When Exyte’s autonomous robotic dog “Rex” came on stage, the dog lover in me briefly expected an actual dog, but instead it was a fully-capable robot that performs laser scanning and reality capture on active construction sites.
It wasn’t a gimmick. It was a signal.
The industry is now designing facilities with unprecedented clarity, speed, and foresight – identifying inefficiencies, and risks before they become expensive, physical problems.
This is exactly where MES belongs as well: Upstream, informing flow, accuracy, operator experience, and automation strategy – not downstream as a corrective measure.
Digital twins may evolve, robots may scan construction progress, but the core truth is unchanged: Execution systems determine how the facility actually runs. They must evolve alongside the facility, not after it.
4. GMP expectations are rising, and digital maturity is becoming synonymous with compliance maturity
Regulatory insights underscored something essential:
GMP expectations now presume data integrity, structured documentation, and robust traceability as default operational conditions.
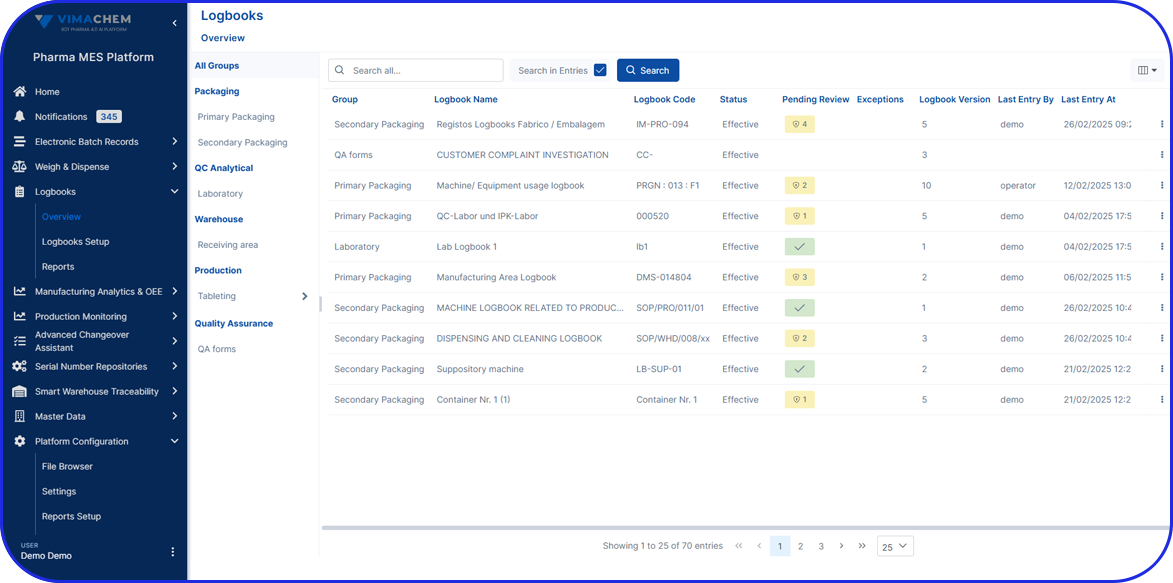
This is not limited to batch records. It spans logbooks, training readiness, environmental evidence, material genealogy, equipment usage, and operator actions.
When these are digitized from the start:
- Investigations shrink.
- Deviations reduce.
- Operator burden decreases.
- Oversight strengthens.
- Consistency becomes engineered.
When they remain manual, the gap between policy and practice widens and regulators increasingly see that as structural risk.
Digital-by-design is fast becoming a compliance-by-design expectation.
5. Talent, training, and operational sustainability demand modular digital systems - not monolithic deployments
Across the sessions, one truth was unmistakable: People are now the limiting factor or the accelerating force in digital manufacturing.
Teams need systems they can learn quickly, adapt rapidly, and trust intuitively.
Today’s realities do not support long, multi-year, monolithic MES deployments:
- Modern workforce expectations.
- Shifting modality requirements.
- Accelerated site start-up schedules.
- Constrained budgets.
- Evolving technology stacks.
A new generation of digital systems is required, one that is both operator-centric and adaptable by design.
This is where modularity, configuration-first architectures, and HITL (human-in-the-loop) principles converge:
- Deploy only what is needed now.
- Expand as processes evolve.
- Upgrade without disruption.
- Configure without code.
- Bring value in increments.
- Support operators without overwhelming them.
- Embed decision support that keeps the human firmly in control.
HITL was repeatedly underscored. Even as AI, automation, and predictive systems mature, operators remain the critical decision-makers, especially in high-variability, high-stakes environments. Digital systems must therefore augment human capability, not replace it. Modular MES, human-centered digitalization is the only model that supports operational sustainability at scale.
6. Why MES now belongs at the heart of facility CAPEX: Vimachem's perspective
Across every theme, a single conclusion emerged. Manufacturing execution is no longer software, it is the operating system of the modern pharma/biopharma site.
This is precisely why MES should be treated as core capital investment, not optional OPEX added late.
Vimachem’s approach aligns with what this next era demands:
MES as modular infrastructure
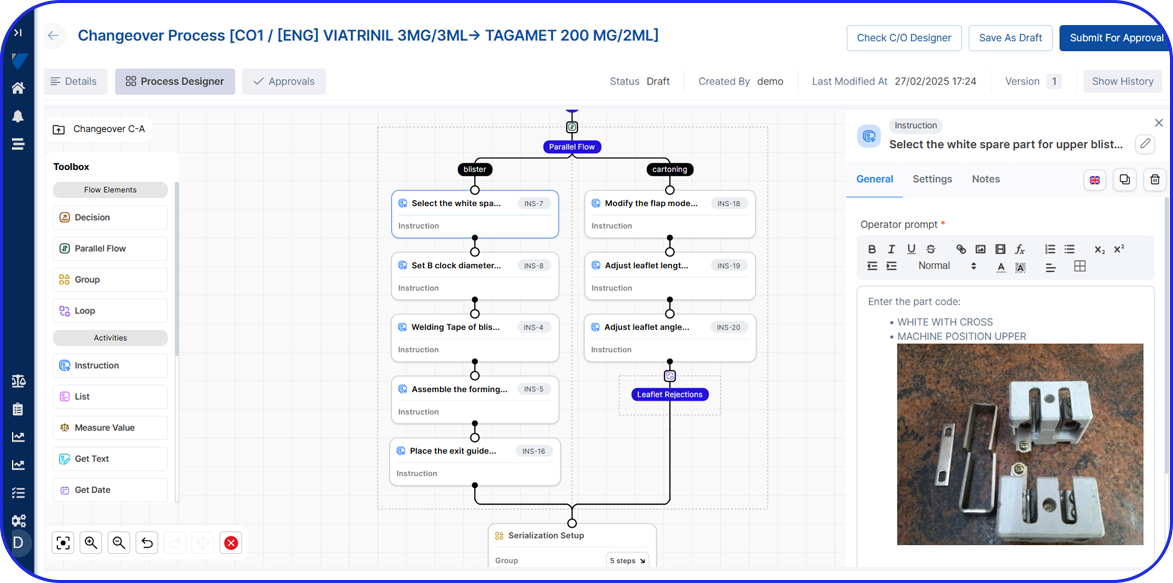
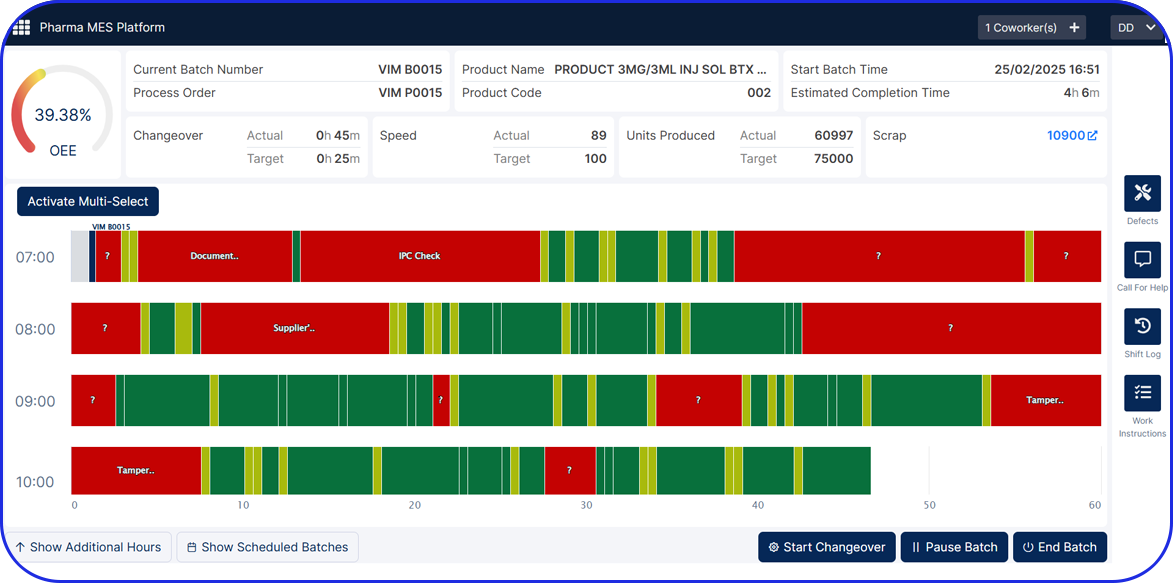
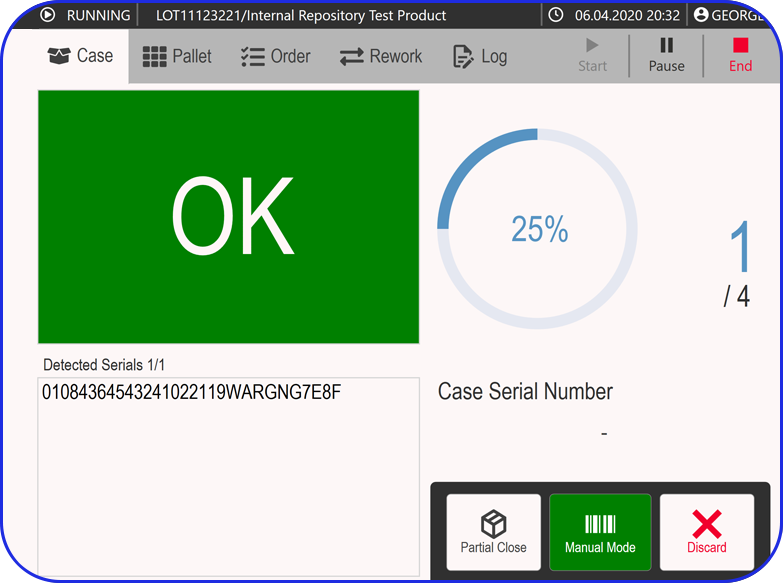
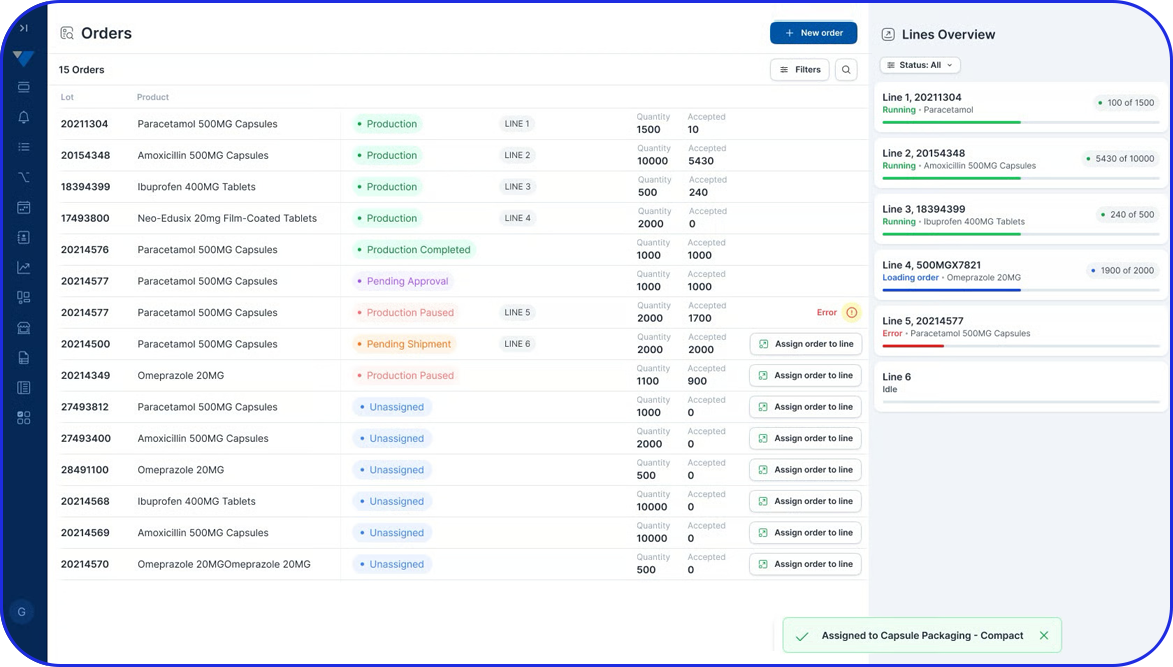
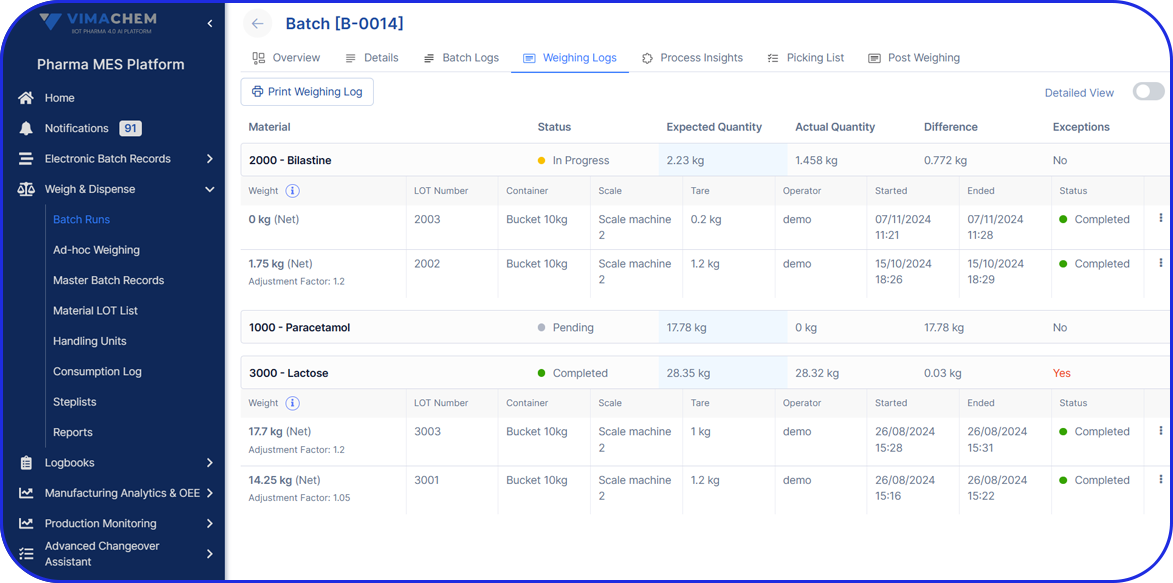
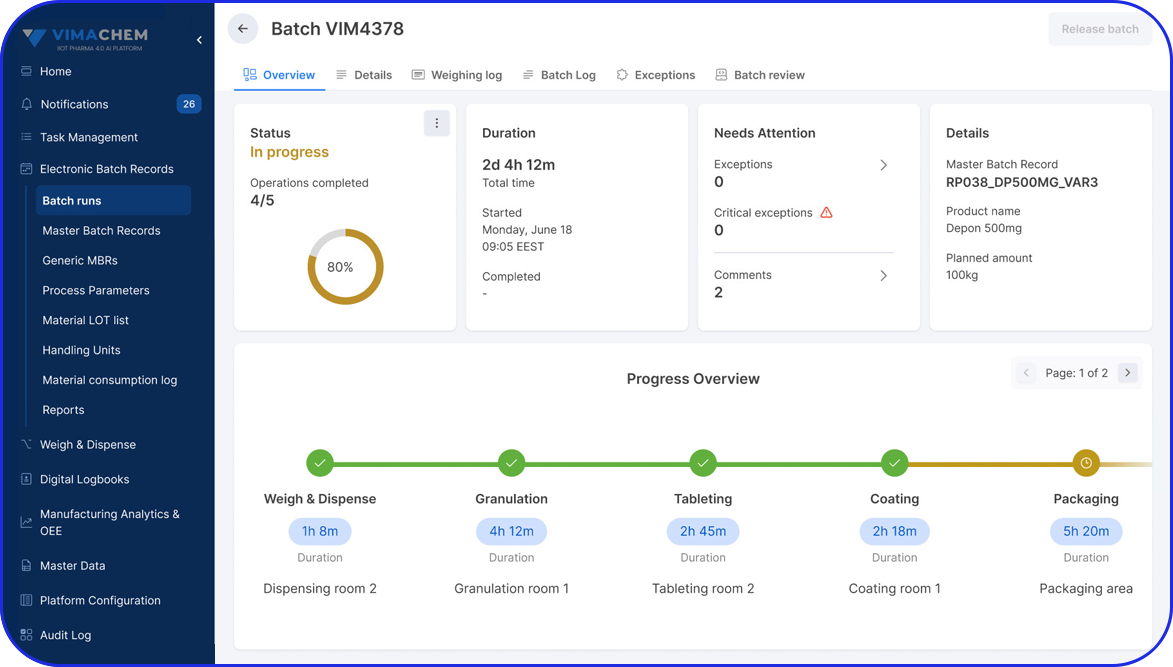
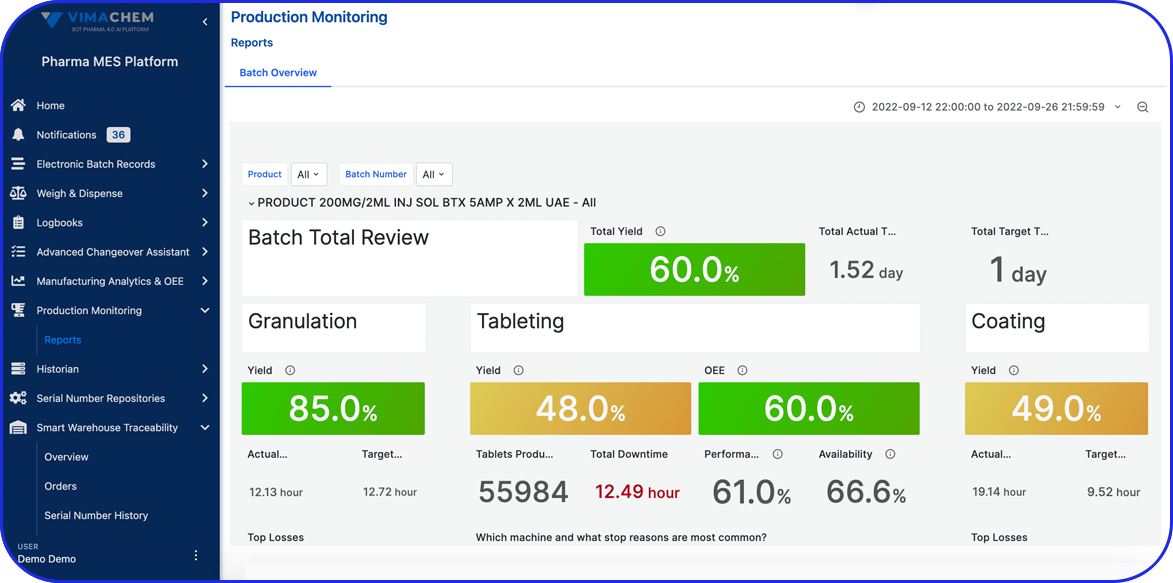
Deployable in discrete components, e.g EBR, Weigh & Dispense, eLogs, DMS, Digital Work Instructions, OEE, Task Management, so sites gain value early and grow without interruption.
MES as design input, not design dependency
Architecture that integrates with facility, equipment, ERP, and automation from day one rather than being forced to retrofit around them.
MES as the bridge between engineering and operations
With digital workflows, material accuracy, automated guidance, structured sequencing, and real-time data as core operational enablers.
MES as the foundation for long-term scalability
Supporting new lines, new modalities, evolving regulatory expectations, and continuous optimization through one easy-to-use, easy-to-maintain, fast-to-deploy platform that drives efficiencies and revenue.
If facilities are being designed with advanced modelling, robotics, modular construction, and precision engineering, the execution layer must evolve in lockstep.
The future state: digital-by-design, modular-by-default, operator-centered by necessity
The next era of pharma & biopharma is not waiting. Pipelines won’t slow. Modalities won’t simplify. Regulatory expectations won’t ease. Capital projects won’t extend timelines.
The winners will be those who recognize that:
- Digital is infrastructure.
- MES is operational backbone.
- Modularity is the only viable approach to complexity.
- Designing digital early is far cheaper than correcting digital late.
- Embedding execution excellence early accelerates outcomes.
Pharma is entering a decisive phase where scientific ambition must be matched with execution intelligence. Building facilities without MES at the core will soon feel as unthinkable as building them without HVAC, utilities, or quality systems.
The next generation of manufacturing will be defined by those who design for digital.
Based on the insights across all talks, the industry’s shift is clear:
- Historically – Digital came after the facility.
- Today – Digital and facility design run in parallel.
- Tomorrow – Facilities will be digital-first by default, with MES embedded as core infrastructure from day 1.