Digitizing Review and Release: Smarter Workflows, Faster Reviews, Stronger Compliance with Vimachem eBR
Digitizing Review and Release: Smarter Workflows, Faster Reviews, Stronger Compliance with Vimachem eBR

Emma Hanley
Senior Product Marketing Manager
In pharma manufacturing, batch review and release can be a bottleneck. Paper-based processes slow production, introduce human error, and delay delivery, while regulators demand precision and proof at every step.
Vimachem’s Electronic Batch Records (eBR) module transforms this process, making review and release faster, more transparent, and inherently compliant.
Live Execution, Instant Visibility
From the Main View, teams can quickly navigate to the eBR workspace and see the full batch lifecycle in one place. The Ops View provides operators with step-by-step instructions, in-process checks, and real-time data capture, all tailored to the Master Batch Record.
Every action is validated as it happens, meaning reviewers aren’t waiting for paperwork at the end of production. By the time a batch reaches review, the data is already accurate, complete, and ready for approval.
In addition to accelerating review, the Vimachem eBR platform empowers manufacturers to monitor critical process and batch parameters in real time. Key checks – such as weight/volume, temperature, pressure, in-process test results, operator sign-offs and deviation flags – are visible to QA and production teams as they happen. This real-time visibility means that non-conformances or deviations can be identified instantly, rather than being discovered only at the end of production. By doing so, the system enables faster corrective action, which reduces the risk of batch re-work, rejected batches or release delays.
This proactive approach turns batch review from a retrospective bottleneck into a live quality assurance activity. With immediate access to execution data and exception alerts, manufacturers can intervene more quickly, mitigate quality events and minimise the cost and impact of non-compliant or out-of-specification (OOS) outcomes.

Smarter Review, Fewer Delays
Instead of review being an afterthought, Vimachem eBR enables concurrent review.
QA teams can:
- Monitor batch execution live.
- Flag and resolve deviations instantly.
- Approve steps without interrupting production.
The Batch Review View consolidates parameters, exceptions, attachments, and signatures into one navigable screen. Review cycles shrink from days to hours, and audit readiness becomes a continuous state rather than a last-minute scramble.
Built-In Compliance from Day One
The eBR module embeds compliance at the point of execution, supporting regulatory requirements under EU GMP (EudraLex Volume 4) (including Annex 11 for computerized systems) and the FDA 21 CFR Part 11 standard on electronic records and signatures.
Highlights include: mandatory validation of critical fields, automated time-stamps and e-signatures, version-controlled instructions, full audit trails, user access control and secure data integrity. These features ensure that electronic batch records are trustworthy, reliable and equivalent to paper records in the eyes of regulators.
Importantly, since the system supports real-time data capture and live release readiness, review and release become continuous activities — significantly shortening the cycle while maintaining rigorous compliance with GMP, 21 CFR Part 11 and Annex 11/Annex 15 expectations.
Scale with Smart Workflow Flexibility
Whether managing a single site or a global network, Vimachem eBR adapts to your environment.
Dynamic workflows automatically adjust instructions, parameter limits, and approval steps based on:
- Batch size.
- Product potency.
- Site-specific requirements.
This flexibility ensures consistent compliance while enabling rapid deployment across facilities.
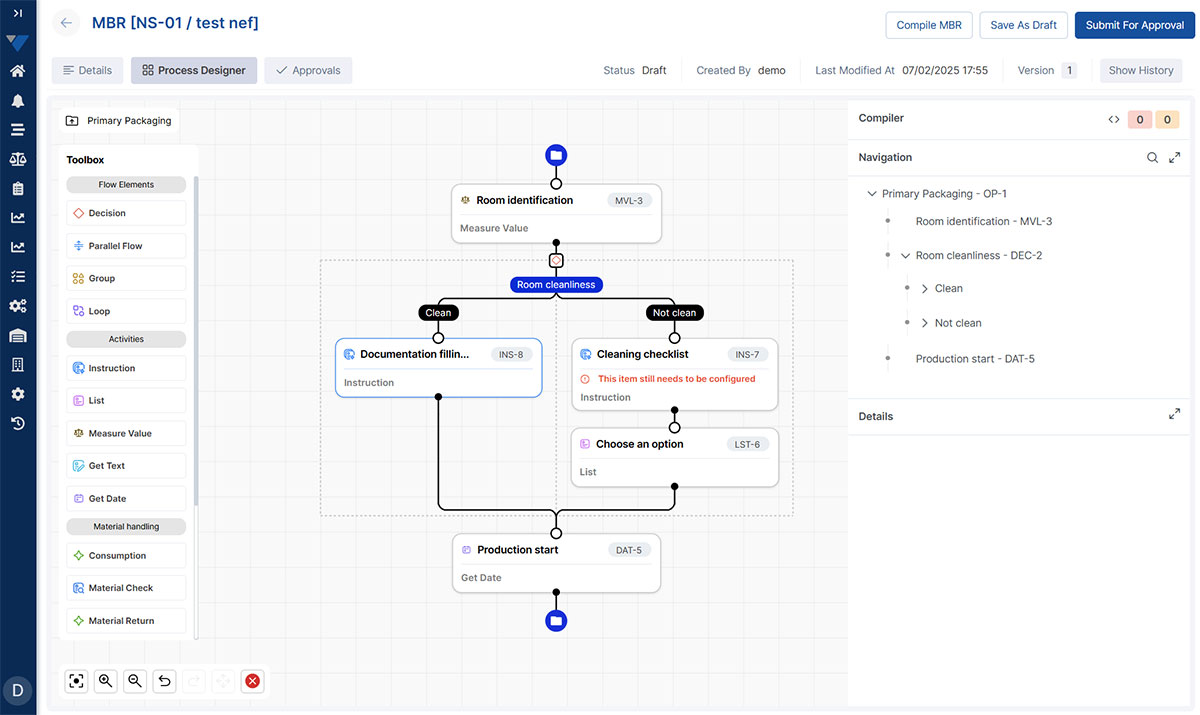
eBR Designer
Part of a Modular MES Platform
Vimachem eBR is part of the AI-driven Modular Pharma MES Platform. Its modular architecture means manufacturers can start with eBR and seamlessly expand into additional modules for greater control, efficiency, and compliance across operations.
Some of the most powerful combinations with eBR include:
- eBR & Weigh & Dispense – Production Operations: Ensures every material is weighed, verified, and automatically linked to the batch record, eliminating manual transcription and guaranteeing traceability from the very first step.
- eBR & Changeover Assistant – Packaging Operations: Speeds and standardizes line changeovers while maintaining full compliance, ensuring packaging runs start on time with minimal risk of error.
- eBR & Weigh & Dispense & Changeover Assistant & Logbooks – Integrated Production & Packaging: Enables the digitization of both batch and non-batch operations across production and packaging, with unified tracking and control from material preparation to final packaging.
By connecting modules, manufacturers can create a fully integrated workflow, from material preparation to packaging, ensuring every action is tracked, validated, and optimized for efficiency. These are just a few examples of how eBR can be combined with other Vimachem modules to deliver end-to-end operational visibility. For the full range of capabilities, explore the MES Platform page.
From Batch Review to Continuous Process Verification
When the eBR module is integrated with equipment-connectivity and machine-data capture modules, you gain the ability to collect equipment critical parameters continuously during each batch run. These may include real-time metrics from process lines (e.g., granulation torque, blend uniformity, coating humidity, tablet hardness) as well as environmental or upstream/downstream equipment data.
This live equipment data can then feed into a Continuous Process Verification (CPV) regime – as endorsed by regulators (for example, the revised EU GMP Annex 15 and the FDA Guidance for Industry: Process Validation – General Principles and Practices).
By leveraging real-time equipment and process data, manufacturers can maintain the process in a validated state across the lifecycle, detect drift or variation trends, and optimise the control strategy rather than simply capturing data post-batch. This means that the eBR becomes not just a tool for review and release, but a platform supporting the lifecycle-based quality framework required under modern GMP.
The result: fewer surprises, better predictive insights, reduced risk of batch failure or delayed release, and stronger regulatory readiness.
A Glimpse at Real-World Impact
A mid-size pharma site running 12 packaging lines reduced their average batch review cycle from three days to less than six hours by implementing Vimachem eBR. Real-time review meant deviations were addressed during execution, avoiding delays and ensuring every record was audit-ready at completion.
Your Path to Digital Review and Release
With Vimachem eBR you move beyond faster review and release. You embed real-time monitoring, early detection of non-conformances, and a platform that supports equipment-integrated controls and life-cycle CPV. In doing so you turn the review-and-release step into a strategic advantage—reducing batch risk, minimising release delays, and enabling robust regulatory compliance under EU GMP and FDA 21 CFR Part 11.
Take the next step: explore how integrated machine-data capture and real-time dashboards can elevate your batch review process into a continuous quality assurance engine. Visit the eBR product page or take a product tour today.
Curious how it fits your operations?
Vimachem’s Pharma MES Platform offers a modular, cutting-edge solution that integrates a machine learning-driven GxP platform with specialized consulting and CSV services, supporting Pharma firms to transform their manufacturing operation in their digital journey to innovation.









